Korean J Pain.
2010 Sep;23(3):179-185. 10.3344/kjp.2010.23.3.179.
Antinociceptive Effect of Memantine and Morphine on Vincristine-induced Peripheral Neuropathy in Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University, Medical School, Gwangju, Korea. leehg@chonnam.ac.kr
- KMID: 1767875
- DOI: http://doi.org/10.3344/kjp.2010.23.3.179
Abstract
- BACKGROUND
Vincristine-induced peripheral neuropathy is a major dose limiting side effect and thus effective therapeutic strategy is required. In this study, we investigated the antinociceptive effect of memantine and morphine on a vincristine-induced peripheral neuropathy model in rats.
METHODS
Male Sprague-Dawley rats weighing 220-240 g were used in all experiments. Rats subsequently received daily intraperitoneal injections of either vincristine sulfate (0.1 ml/kg/day) or saline (0.1 ml/kg/day) over 12 days, immediately following behavioral testing. For assessment of mechanical allodynia, mechanical stimuli using von Frey filament was applied to the paw to measure withdrawal threshold. The effects of N-methyl-D-aspartate receptors antagonist (memantine; 2.5, 5, 10 mg/kg intraperitoneal), opioid agonist (morphine; 2.5, 5, 10 mg/kg intraperitoneal) and vehicle (saline) on vicristine-induced neuropathy were evaluated.
RESULTS
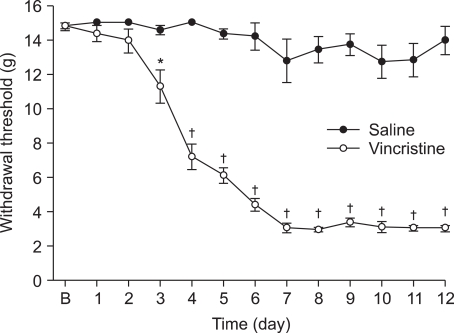
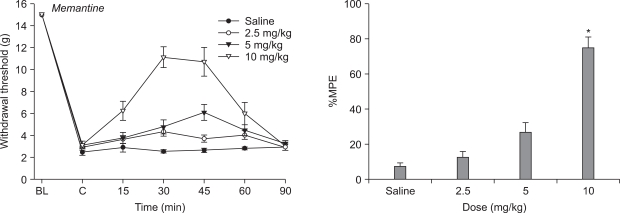
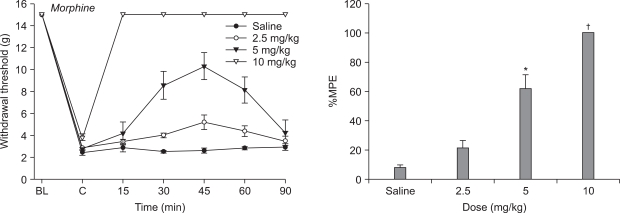
Mechanical allodynia developed over the course of ten daily injections of vincristine relative to groups receiving saline at the same time. Morphine abolished the reduction in paw withdrawal threshold compared to vehicle and produced dose-responsiveness. Only the highest dose of memantine (10 mg/kg) was able to increase paw withdrawal threshold compared to vehicle.
CONCLUSIONS
Systemic morphine and memantine have an antinociceptive effect on the vincristine-induced peripheral neuropathy model in rats. These results suggest morphine and memantine may be an alternative approach for the treatment of vincristine-induced peripheral neuropathic pain.
Keyword
MeSH Terms
Figure
Reference
-
1. Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002; 249:9–17. PMID: 11954874.
Article2. Postma TJ, Benard BA, Huijgens PC, Ossenkoppele GJ, Heimans JJ. Long-term effects of vincristine on the peripheral nervous system. J Neurooncol. 1993; 15:23–27. PMID: 8384253.3. Himes RH, Kersey RN, Heller-Bettinger I, Samson FE. Action of the vinca alkaloids vincristine, vinblastine, and desacetyl vinblastine amide on microtubules in vitro. Cancer Res. 1976; 36:3798–3802. PMID: 954003.4. Owellen RJ, Hartke CA, Dickerson RM, Hains FO. Inhibition of tubulin-microtubule polymerization by drugs of the Vinca alkaloid class. Cancer Res. 1976; 36:1499–1502. PMID: 1260766.5. Casey EB, Jellife AM, Le Quesne PM, Millett YL. Vincristine neuropathy. Clinical and electrophysiological observations. Brain. 1973; 96:69–86. PMID: 4348690.6. Weiden PL, Wright SE. Vincristine neurotoxicity. N Engl J Med. 1972; 286:1369–1370. PMID: 5027400.
Article7. Forman A. Peripheral neuropathy in cancer patients: clinical types, etiology, and presentation. Part 2. Oncology (Williston Park). 1990; 4:85–89. PMID: 2167114.8. Sandler SG, Tobin W, Henderson ES. Vincristine-induced neuropathy. A clinical study of fifty leukemic patients. Neurology. 1969; 19:367–374. PMID: 5813374.
Article9. Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008; 44:1507–1515. PMID: 18571399.
Article10. Ogawa T, Mimura Y, Kato H, Ootsubo S, Murakoshi M. The usefulness of rabbits as an animal model for the neuropathological assessment of neurotoxicity following the administration of vincristine. Neurotoxicology. 2000; 21:501–511. PMID: 11022859.11. Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000; 424:563–576. PMID: 10931481.
Article12. Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003; 103:131–138. PMID: 12749967.
Article13. Lynch JJ 3rd, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004; 110:56–63. PMID: 15275752.
Article14. Kaley TJ, Deangelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol. 2009; 145:3–14. PMID: 19170681.
Article15. Arnér S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988; 33:11–23. PMID: 2454440.
Article16. Dellemijn P. Are opioids effective in relieving neuropathic pain? Pain. 1999; 80:453–462. PMID: 10342407.
Article17. Obara I, Makuch W, Spetea M, Schütz J, Schmidhammer H, Przewlocki R, et al. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur J Pharmacol. 2007; 558:60–67. PMID: 17204264.
Article18. Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA. 2005; 293:3043–3052. PMID: 15972567.
Article19. Chizh BA, Headley PM. NMDA antagonists and neuropathic pain--multiple drug targets and multiple uses. Curr Pharm Des. 2005; 11:2977–2994. PMID: 16178757.
Article20. Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000; 4:5–15. PMID: 10833550.
Article21. Buvanendran A, Kroin JS. Early use of memantine for neuropathic pain. Anesth Analg. 2008; 107:1093–1094. PMID: 18806007.
Article22. Finkel JC, Pestieau SR, Quezado ZM. Ketamine as an adjuvant for treatment of cancer pain in children and adolescents. J Pain. 2007; 8:515–521. PMID: 17434801.
Article23. Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004; 1:101–110. PMID: 15717010.
Article24. Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997; 280:829–838. PMID: 9023297.25. Zimmermann M. Ethical guidelines for investigations on experimental pain in conscious animals. Pain. 1983; 16:109–110. PMID: 6877845.
Article26. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.
Article27. Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006; 72:151–169. PMID: 16493391.28. Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001; 2:8–14. PMID: 15102312.
Article29. Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007; 152:765–777. PMID: 17572696.
Article30. Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine Study Group. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003; 348:1333–1341. PMID: 12672860.
Article31. Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004; 291:317–324. PMID: 14734594.
Article32. Sinis N, Birbaumer N, Gustin S, Schwarz A, Bredanger S, Becker ST, et al. Memantine treatment of complex regional pain syndrome: a preliminary report of six cases. Clin J Pain. 2007; 23:237–243. PMID: 17314583.33. Hackworth RJ, Tokarz KA, Fowler IM, Wallace SC, Stedje-Larsen ET. Profound pain reduction after induction of memantine treatment in two patients with severe phantom limb pain. Anesth Analg. 2008; 107:1377–1379. PMID: 18806054.
Article34. Carlton SM, Hargett GL. Treatment with the NMDA antagonist memantine attenuates nociceptive responses to mechanical stimulation in neuropathic rats. Neurosci Lett. 1995; 198:115–118. PMID: 8592634.
Article35. Suzuki R, Matthews EA, Dickenson AH. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain. 2001; 91:101–109. PMID: 11240082.
Article36. Medvedev IO, Malyshkin AA, Belozertseva IV, Sukhotina IA, Sevostianova NY, Aliev K, et al. Effects of low-affinity NMDA receptor channel blockers in two rat models of chronic pain. Neuropharmacology. 2004; 47:175–183. PMID: 15223296.
Article37. Bulka A, Plesan A, Xu XJ, Wiesenfeld-Hallin Z. Reduced tolerance to the anti-hyperalgesic effect of methadone in comparison to morphine in a rat model of mononeuropathy. Pain. 2002; 95:103–109. PMID: 11790472.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Effect of Intrathecal Nefopam and Interaction with Morphine in Formalin-Induced Pain of Rats
- Effect of intrathecal oxcarbazepine on rat tail flick test-determined morphine tolerance
- Supraspinal Nitric Oxide Synthesis Inhibition Enhanced Antinociception of Morphine in Morphine Tolerant Rats
- Memantine Reverses Social Withdrawal Induced by Ketamine in Rats
- Intrathecal lamotrigine blocks and reverses antinociceptive morphine tolerance in rats