Yonsei Med J.
2009 Aug;50(4):474-481. 10.3349/ymj.2009.50.4.474.
Comparison of the Effects of Alendronate and Alfacalcidol on Hip Bone Mineral Density and Bone Turnover in Japanese Men Having Osteoporosis or Osteopenia with Clinical Risk Factors for Fractures
- Affiliations
-
- 1Institute for Integrated Sports Medicine, Keio University School of Medicine, Tokyo, Japan. jiwamoto@sc.itc.keio.ac.jp
- 2Department of Neurology, Mitate Hospital, Fukuoka, Japan.
- 3Department of Orthopaedic Surgery, Keiyu Orthopaedic Hospital, Gunma, Japan.
- KMID: 1758605
- DOI: http://doi.org/10.3349/ymj.2009.50.4.474
Abstract
- PURPOSE
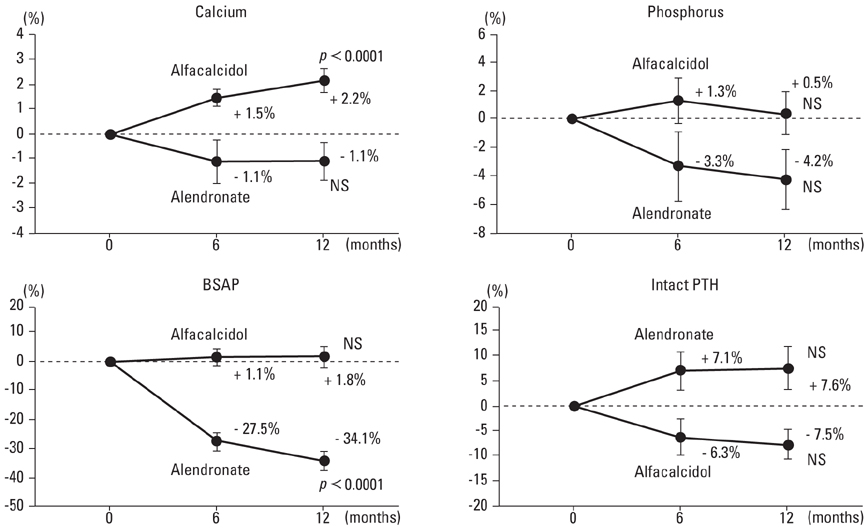
The comparative effects of alendronate and alfacalcidol on bone mineral density (BMD) and bone turnover have already been established in postmenopausal women with osteoporosis. An open-labeled prospective study was conducted to compare the treatment effects of alendronate and alfacalcidol on hip BMD and bone turnover in Japanese men with osteoporosis or osteopenia with clinical risk factors for fractures. MATERIALS AND METHODS: One hundred twelve men with osteoporosis or osteopenia with clinical risk factors for fractures (mean age: 71.4 years) were randomly divided into two groups of 56 patients each: the alendronate (5 mg daily) and alfacalcidol (1 microgram daily) groups. The BMD of the total hip, urinary level of cross-linked N-terminal telopeptides of type I collagen (NTX), and serum levels of bone-specific alkaline phosphatase (BSAP) were measured during the 12-month-treatment period. RESULTS: Forty-five patients in the alendronate group and 42 patients in the alfacalcidol group completed the trial. Alendronate increased BMD (+2.3% at 12 months) following reductions in the urinary level of NTX (-46.4% at 3 months) and serum level of BSAP (-34.1% at 12 months), while alfacalcidol sustained BMD (-1.9% at 12 months) as well as the urinary level of NTX (+13.2% at 3 months) and serum level of BSAP (+1.8% at 12 months). CONCLUSION: The present study confirmed that alendronate has better efficacy than alfacalcidol (active control) in increasing hip BMD and reducing bone turnover in Japanese men with osteoporosis or osteopenia with clinical risk factors for fractures.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Alendronate/pharmacology/therapeutic use
Asian Continental Ancestry Group
Bone Density/*drug effects
*Bone Density Conservation Agents/pharmacology/therapeutic use
Bone Diseases, Metabolic/*drug therapy
Fractures, Bone/*prevention & control
Hip Joint/*drug effects/pathology
Humans
*Hydroxycholecalciferols/pharmacology/therapeutic use
Male
Middle Aged
Osteoporosis/*drug therapy
Treatment Outcome
Figure
Reference
-
1. Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995. 333:1437–1443.
Article2. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996. 348:1535–1541.3. Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998. 280:2077–2082.
Article4. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004. 350:1189–1199.
Article5. Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006. 296:2927–2938.
Article6. Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C. Osteoporsis Methodology Group. The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002. 23:570–578.
Article7. Papapoulos SE, Quandt SA, Liberman UA, Hochberg MC, Thompson DE. Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int. 2005. 16:468–474.
Article8. Liberman UA, Hochberg MC, Geusens P, Shah A, Lin J, Chattopadhyay A, et al. Hip and non-spine fracture risk reductions differ among antiresorptive agents: Evidence from randomised controlled trials. Int J Clin Pract. 2006. 60:1394–1400.
Article9. Hochberg MC, Thompson DE, Black DM, Quandt SA, Cauley J, Geusens P, et al. Effect of alendronate on the age-specific incidence of symptomatic osteoporotic fractures. J Bone Miner Res. 2005. 20:971–976.
Article10. Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000. 343:604–610.
Article11. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003. 32:468–473.
Article12. Tsuboi M, Hasegawa Y, Suzuki S, Wingstrand H, Thorngren KG. Mortality and mobility after hip fracture in Japan: a ten-year follow-up. J Bone Joint Surg Br. 2007. 89:461–466.13. Hasegawa Y, Suzuki S, Wingstrand H. Risk of mortality following hip fracture in Japan. J Orthop Sci. 2007. 12:113–117.
Article14. Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002. 87:1586–1592.
Article15. Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004. 19:1250–1258.
Article16. Ringe JD, Orwoll E, Daifotis A, Lombardi A. Treatment of male osteoporosis: recent advances with alendronate. Osteoporos Int. 2002. 13:195–199.
Article17. Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006. 26:427–431.
Article18. Ringe JD, Farahmand P, Schacht E, Rozehnal A. Superiority of a combined treatment of Alendronate and Alfacalcidol compared to the combination of Alendronate and plain vitamin D or Alfacalcidol alone in established postmenopausal or male osteoporosis (AAC-Trial). Rheumatol Int. 2007. 27:425–434.
Article19. Gonnelli S, Cepollaro C, Montagnani A, Bruni D, Caffarelli C, Breschi M, et al. Alendronate treatment in men with primary osteoporosis: a three-year longitudinal study. Calcif Tissue Int. 2003. 73:133–139.
Article20. Ringe JD, Dorst A, Faber H, Ibach K. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004. 24:110–113.
Article21. Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, et al. Diagnostic criteria of primary osteoporosis. J Bone Miner Metab. 1998. 16:139–150.
Article22. Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001. 19:331–337.
Article23. Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, et al. Assessment of fracture risk. Osteoporos Int. 2005. 16:581–589.
Article24. Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, et al. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporos Int. 1999. 10:183–192.
Article25. Kushida K, Shiraki M, Nakamura T, Kishimoto H, Morii H, Yamamoto K, et al. The efficacy of alendronate in reducing the risk for vertebral fracture in Japanese patients with osteoporosis: a randomized, double-blind, active-controlled, double-dummy trial. Curr Ther Res. 2002. 63:606–620.
Article26. Orimo H, Shiraki M, Hayashi Y, Hoshino T, Onaya T, Miyazaki S, et al. Effects of 1 alpha-hydroxyvitamin D3 on lumbar bone mineral density and vertebral fractures in patients with postmenopausal osteoporosis. Calcif Tissue Int. 1994. 54:370–376.
Article27. Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, et al. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J Bone Miner Metab. 2005. 23:97–104.
Article28. Seeman E. Unresolved issues in osteoporosis in men. Rev Endocr Metab Disord. 2001. 2:45–64.29. Resch H, Pietschmann P, Woloszczuk W, Krexner E, Bernecker P, Willvonseder R. Bone mass and biochemical parameters of bone metabolism in men with spinal osteoporosis. Eur J Clin Invest. 1992. 22:542–545.
Article30. Sharp CA, Worsfold M, Rowlands PR, Davie MWJ. Accurate prediction of spinal osteoporosis in men using a biochemical measure of collagen balance. Bone. 1994. 15:243.
Article31. Shiraishi A, Takeda S, Masaki T, Higuchi Y, Uchiyama Y, Kubodera N, et al. Alfacalcidol inhibits bone resorption and stimulates formation in an ovariectomized rat model of osteoporosis: distinct actions from estrogen. J Bone Miner Res. 2000. 15:770–779.
Article32. Schacht E. Rationale for treatment of involutional osteoporosis in women and for prevention and treatment of corticosteroid-induced osteoporosis with alfacalcidol. Calcif Tissue Int. 1999. 65:317–327.
Article33. Shiraishi A, Higashi S, Masaki T, Saito M, Ito M, Ikeda S, et al. A comparison of alfacalcidol and menatetrenone for the treatment of bone loss in an ovariectomized rat model of osteoporosis. Calcif Tissue Int. 2002. 71:69–79.
Article34. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004. 291:1999–2006.35. Jesudason D, Need AG, Horowitz M, O'Loughlin PD, Morris HA, Nordin BE. Relationship between serum 25-hydroxy vitamin D and bone resorption markers in vitamin D insufficiency. Bone. 2002. 31:626–630.
Article36. de Nijs RN, Jacobs JW, Lems WF, Laan RF, Algra A, Huisman AM, et al. Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med. 2006. 355:675–684.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Paper “Comparison of the Effects of Alendronate and Alfacalcidol on Hip Bone Mineral Density and Bone Turnover in Japanese Men Having Osteoporosis or Osteopenia with Clinical Risk Factors for Fractures” by Iwamoto J, et al. [Yonsei Med J 2009 Aug;50(4):474-481]
- Osteoporosis and Prevalent Fractures among Adult Filipino Men Screened for Bone Mineral Density in a Tertiary Hospital
- The Effects of Alendronate in Bone Metabolism of Primary Osteoporosis
- Persistency and Change of the Bone Mineral Density with Alendronate Treatment after Hip Fracture
- Pharmacological Therapy for Postmenopausal Osteoporosis