Clin Orthop Surg.
2011 Sep;3(3):230-237. 10.4055/cios.2011.3.3.230.
Disturbed Osteoblastic Differentiation of Fibrous Hamartoma Cell from Congenital Pseudarthrosis of the Tibia Associated with Neurofibromatosis Type I
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul National University College of Medicine, Seoul, Korea. tjcho@snu.ac.kr
- KMID: 1743914
- DOI: http://doi.org/10.4055/cios.2011.3.3.230
Abstract
- BACKGROUND
Fibrous hamartoma is the key pathology of congenital pseudarthrosis of the tibia (CPT), which was shown to have low osteogenicity and high osteoclastogenicity. This study further investigated the mechanism of impaired osteoblastic differentiation of fibrous hamartoma cells.
METHODS
Fibroblast-like cells were obtained from enzymatically dissociated fibrous hamartomas of 11 patients with CPT associated with neurofibromatosis type I (NF1). Periosteal cells were also obtained from the distal tibial periosteum of 3 patients without CPT or NF1 as control. The mRNA levels of Wnt ligands and their canonical receptors, such as Lrp5 and beta-catenin, were assayed using reverse transcriptase PCR (RT-PCR). Changes in mRNA expression of osteoblast marker genes by rhBMP2 treatment were assayed using quantitative real time RT-PCR. Changes in mRNA expression of transcription factors specifically involved in osteoblastic differentiation by rhBMP2 treatment was also assayed using quantitative real-time RT-PCR.
RESULTS
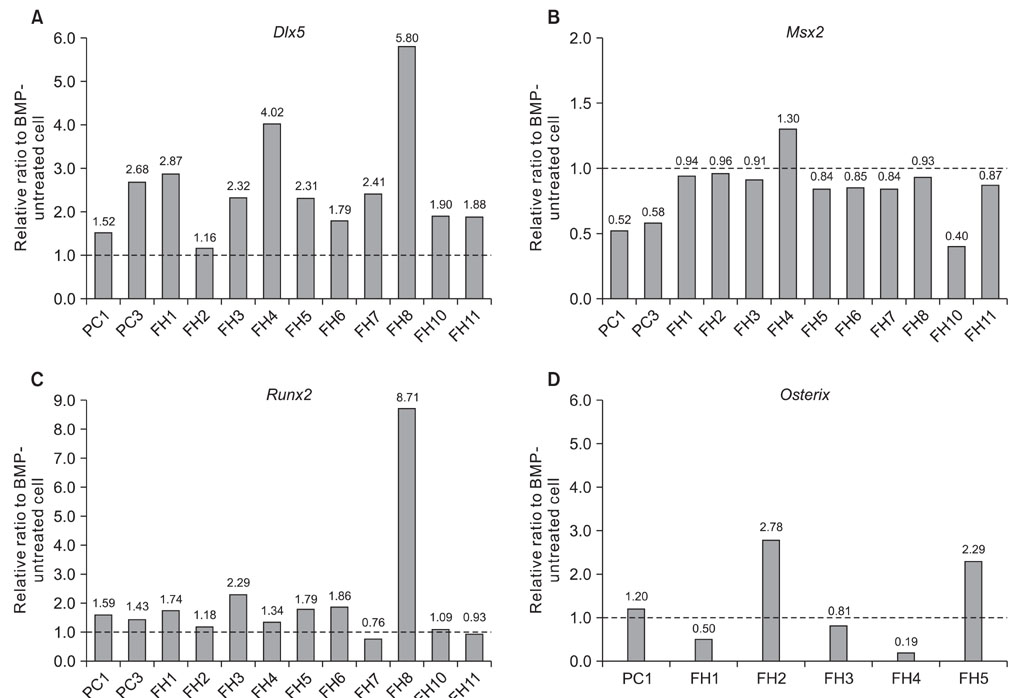
Wnt1 and Wnt3a mRNA expression was lower in fibrous hamartoma than in tibial periosteal cells, but their canonical receptors did not show significant difference. Response of osteoblastic marker gene expression to rhBMP2 treatment showed patient-to-patient variability. Col1a1 mRNA expression was up-regulated in most fibrous hamartoma tissues, osteocalcin was up-regulated in a small number of patients, and ALP expression was down-regulated in most fibrous hamartoma tissues. Changes in mRNA expression of the transcription factors in response to rhBMP2 also showed factor-to-factor and patient-to-patient variability. Dlx5 was consistently up-regulated by rhBMP2 treatment in all fibrous hamartoma tissues tested. Msx2 expression was down-regulated by rhBMP2 in most cases but by lesser extent than control tissue. Runx2 expression was up-regulated in 8 out of 18 fibrous hamartoma tissues tested. Osterix expression was up-regulated in 2 and down-regulated in 3 fibrous hamartoma tissues.
CONCLUSIONS
Congenital pseudarthrosis of the tibia appears to be caused by fibrous hamartoma originating from aberrant growth of Nf1 haploinsufficient periosteal cells, which failed in terminal osteoblastic differentiation and arrested at a certain stage of this process. This pathomechanism of CPT should be targeted in the development of novel therapeutic biologic intervention.
MeSH Terms
-
Adolescent
*Cell Differentiation
Cells, Cultured
Child
Child, Preschool
Female
Hamartoma/complications/*pathology
Humans
Infant
Low Density Lipoprotein Receptor-Related Protein-5/metabolism
Male
Neurofibromatosis 1/complications/*pathology
Osteoblasts/*pathology
Periosteum/pathology
Pseudarthrosis/complications/*congenital/pathology/physiopathology
Receptors, Wnt/metabolism
Reverse Transcriptase Polymerase Chain Reaction
Tibia/*pathology
Transcription Factors/metabolism
Wnt1 Protein/metabolism
Wnt3A Protein/metabolism
beta Catenin/metabolism
Figure
Reference
-
1. Ippolito E, Corsi A, Grill F, Wientroub S, Bianco P. Pathology of bone lesions associated with congenital pseudarthrosis of the leg. J Pediatr Orthop B. 2000. 9(1):3–10.
Article2. Hefti F, Bollini G, Dungl P, et al. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop B. 2000. 9(1):11–15.
Article3. Crawford AH, Schorry EK. Neurofibromatosis update. J Pediatr Orthop. 2006. 26(3):413–423.
Article4. Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999. 89(1):1–6.
Article5. Shen MH, Harper PS, Upadhyaya M. Molecular genetics of neurofibromatosis type 1 (NF1). J Med Genet. 1996. 33(1):2–17.
Article6. Gutmann DH, Wood DL, Collins FS. Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci U S A. 1991. 88(21):9658–9662.
Article7. Kuorilehto T, Nissinen M, Koivunen J, Benson MD, Peltonen J. NF1 tumor suppressor protein and mRNA in skeletal tissues of developing and adult normal mouse and NF1-deficient embryos. J Bone Miner Res. 2004. 19(6):983–989.
Article8. Leskela HV, Kuorilehto T, Risteli J, et al. Congenital pseudarthrosis of neurofibromatosis type 1: impaired osteoblast differentiation and function and altered NF1 gene expression. Bone. 2009. 44(2):243–250.
Article9. Bollag G, Clapp DW, Shih S, et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet. 1996. 12(2):144–148.
Article10. Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001. 104(4):593–604.11. Schindeler A, Little DG. Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NF1). Bone. 2008. 42(4):616–622.
Article12. Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994. 7(3):353–361.
Article13. Cichowski K, Shih TS, Schmitt E, et al. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999. 286(5447):2172–2176.
Article14. Upadhyaya M, Han S, Consoli C, et al. Characterization of the somatic mutational spectrum of the neurofibromatosis type 1 (NF1) gene in neurofibromatosis patients with benign and malignant tumors. Hum Mutat. 2004. 23(2):134–146.
Article15. Li Y, Bollag G, Clark R, et al. Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell. 1992. 69(2):275–281.
Article16. Cho TJ, Seo JB, Lee HR, Yoo WJ, Chung CY, Choi IH. Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1. J Bone Joint Surg Am. 2008. 90(12):2735–2744.
Article17. Mariaud-Schmidt RP, Rosales-Quintana S, Bitar E, et al. Hamartoma involving the pseudarthrosis site in patients with neurofibromatosis type 1. Pediatr Dev Pathol. 2005. 8(2):190–196.
Article18. Chen Y, Alman BA. Wnt pathway, an essential role in bone regeneration. J Cell Biochem. 2009. 106(3):353–362.
Article19. Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006. 7(1-2):33–39.
Article20. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997. 89(5):747–754.
Article21. Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006. 366(1):51–57.
Article22. Ryoo HM, Hoffmann HM, Beumer T, et al. Stage-specific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol Endocrinol. 1997. 11(11):1681–1694.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Characterization of Congenital Pseudoarthrosis of the Tibia Associated with Neurofibromatosis
- Congenital Pseudarthrosis of Bones of the Forearm: A Case Report
- Congenital Pseudarthrosis of the Tibia
- A Case Congenital Pseudarthrosis of Tibia
- Electrical Stimulation ofCongenital Pseudarthrosis of the Tibia: a case report