J Vet Sci.
2014 Mar;15(1):111-115. 10.4142/jvs.2014.15.1.111.
Mouse Fyn induces pseudopodium formation in Chinese hamster ovary cells
- Affiliations
-
- 1College of Veterinary Medicine, Northwest A&F University, Yangling 712100, China. shantingzhao@hotmail.com
- KMID: 1737616
- DOI: http://doi.org/10.4142/jvs.2014.15.1.111
Abstract
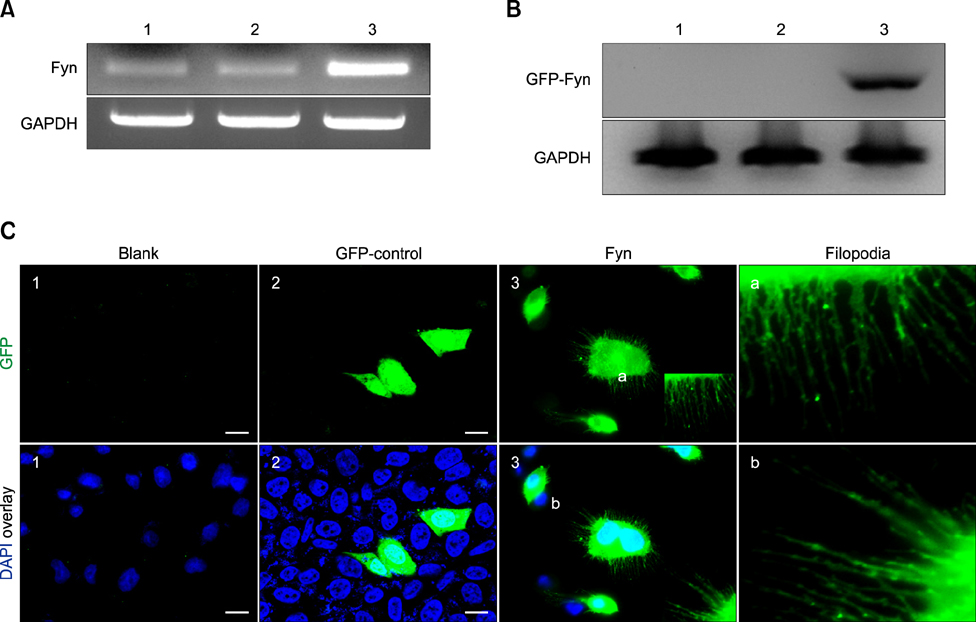
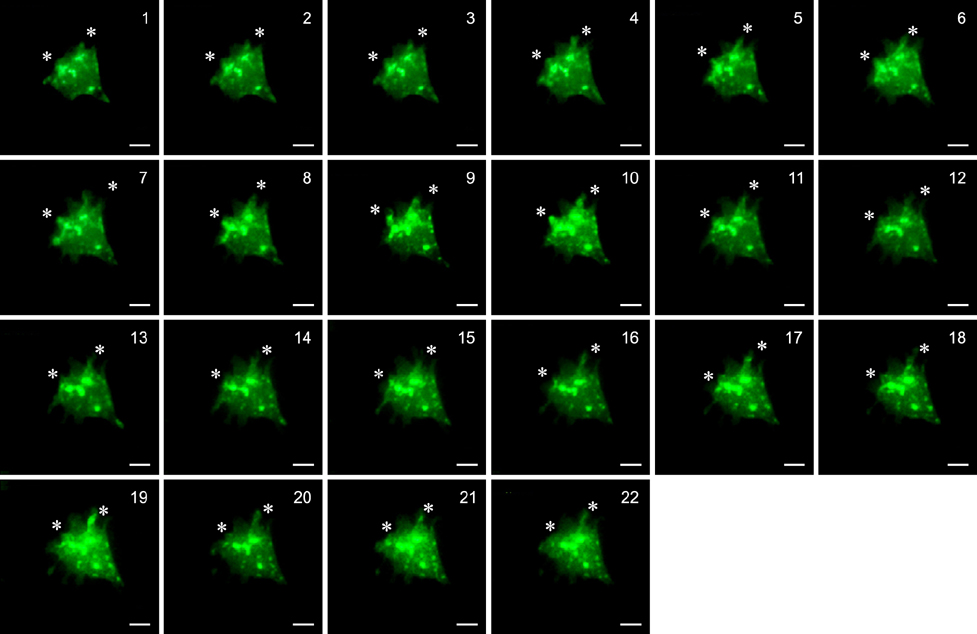
- Molecular mechanisms underlying the effects of Fyn on cell morphology, pseudopodium movement, and cell migration were investigated. The Fyn gene was subcloned into pEGFP-N1 to produce pEGFP-N1-Fyn. Chinese hamster ovary (CHO) cells were transfected with pEGFP-N1-Fyn. The expression of Fyn mRNA and proteins was monitored by reverse transcription-PCR and Western blotting. Additionally, transfected cells were stained with 4',6-diamidino-2-phenylindole and a series of time-lapse images was taken. Sequences of the recombinant plasmids pMD18-T-Fyn and pEGFP-N1-Fyn were confirmed by sequence identification using National Center for Biotechnology Information in USA, and Fyn expression was detected by RT-PCR and Western blotting. The morphology of CHO cells transfected with the recombinant vector was significantly altered. Fyn expression induced filopodia and lamellipodia formation. Based on these results, we concluded that overexpression of mouse Fyn induces the formation of filopodia and lamellipodia in CHO cells, and promotes cell movement.
Keyword
MeSH Terms
-
Animals
Blotting, Western
CHO Cells
Cricetinae
Cricetulus
Genetic Vectors
Green Fluorescent Proteins/genetics
Mice
Proto-Oncogene Proteins c-fyn/genetics/*metabolism
Pseudopodia/*metabolism
Reverse Transcriptase Polymerase Chain Reaction
Time-Lapse Imaging
Transfection
Green Fluorescent Proteins
Proto-Oncogene Proteins c-fyn
Figure
Reference
-
1. Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003; 13:9–17.
Article2. Bristow JM, Reno TA, Jo M, Gonias SL, Klemke RL. Dynamic phosphorylation of tyrosine 665 in pseudopodium-enriched atypical kinase 1 (PEAK1) is essential for the regulation of cell migration and focal adhesion turnover. J Biol Chem. 2013; 288:123–131.
Article3. Buel GR, Rush J, Ballif BA. Fyn promotes phosphorylation of collapsin response mediator protein 1 at tyrosine 504, a novel, isoform-specific regulatory site. J Cell Biochem. 2010; 111:20–28.
Article4. Chai X, Förster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci. 2009; 29:288–299.
Article5. Frotscher M. Role for Reelin in stabilizing cortical architecture. Trends Neurosci. 2010; 33:407–414.
Article6. Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998; 279:509–514.
Article7. Kim YB, Choi S, Choi MC, Oh MA, Lee SA, Cho M, Mizuno K, Kim SH, Lee JW. Cell adhesion-dependent cofilin serine 3 phosphorylation by the integrin-linked kinase·c-Src complex. J Biol Chem. 2008; 283:10089–10096.
Article8. Li W, Marshall C, Mei L, Gelfand J, Seykora JT. Srcasm corrects Fyn-induced epidermal hyperplasia by kinase down-regulation. J Biol Chem. 2007; 282:1161–1169.
Article9. Lesslie DP 3rd, Summy JM, Parikh NU, Fan F, Trevino JG, Sawyer TK, Metcalf CA 3rd, Shakespeare WC, Hicklin DJ, Ellis LM, Gallick GE. Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. Br J Cancer. 2006; 94:1710–1717.
Article10. Long H, Bock HH, Lei T, Chai X, Yuan J, Herz J, Frotscher M, Yang Z. Identification of alternatively spliced Dab1 and Fyn isoforms in pig. BMC Neurosci. 2011; 12:17.
Article11. Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998; 111:3167–3177.
Article12. Marie-Cardine A, Kirchgessner H, Eckerskorn C, Meuer SC, Schraven B. Human T lymphocyte activation induces tyrosine phosphorylation of α-tubulin and its association with the SH2 domain of the p59fyn protein tyrosine kinase. Eur J Immunol. 1995; 25:3290–3297.
Article13. Ninio-Many L, Grossman H, Shomron N, Chuderland D, Shalgi R. microRNA-125a-3p reduces cell proliferation and migration by targeting Fyn. J Cell Sci. 2013; 126:2867–2876.14. Suetsugu S, Hattori M, Miki H, Tezuka T, Yamamoto T, Mikoshiba K, Takenawa T. Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev Cell. 2002; 3:645–658.
Article15. Wang Y, Kelber JA, Tran Cao HS, Cantin GT, Lin R, Wang W, Kaushal S, Bristow JM, Edgington TS, Hoffman RM, Bouvet M, Yates JR 3rd, Klemke RL. Pseudopodium-enriched atypical kinase 1 regulates the cytoskeleton and cancer progression. Proc Natl Acad Sci U S A. 2010; 107:10920–10925.
Article16. Yeo MG, Oh HJ, Cho HS, Chun JS, Marcantonio EE, Song WK. Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J Cell physiol. 2011; 226:236–247.
Article17. Yadav V, Denning MF. Fyn is induced by Ras/PI3K/Akt signaling and is required for enhanced invasion/migration. Mol Carcinog. 2011; 50:346–352.
Article18. Zamora-Leon SP, Shafit-Zagardo B. Disruption of the actin network enhances MAP-2c and Fyn-induced process outgrowth. Cell Motil Cytoskeleton. 2005; 62:110–123.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Induction of Sister-Chromadd Exchanges in Chinese Hamster Ovary Kl Cells by Exposure to Cadmium
- Cloning and expression of rat liver type glucose transporter and translocation by insulin in Chinese hamster ovary cells
- Production and Characterization of Human CD27lg, CD40fg and CD95lg Fusion Proteins in Chinese Hamster Ovary Cell
- Expression of HBsAg Containing the PreS1, PreS2 and S in Chinese Hamster Ovary Cell
- Fyn Tyrosine Kinase-mediated Tyrosine Phosphorylation of Roundabout (Robo), the Slit Receptor