Korean J Lab Med.
2011 Jul;31(3):131-137. 10.3343/kjlm.2011.31.3.131.
Reliable, Accurate Determination of the Leukocyte Differential of Leukopenic Samples by Using Hematoflow Method
- Affiliations
-
- 1Department of Laboratory Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. hankja@catholic.ac.kr
- KMID: 1735842
- DOI: http://doi.org/10.3343/kjlm.2011.31.3.131
Abstract
- BACKGROUND
Hematology analyzers may ineffectively recognize abnormal cells, and manual differential counts may be imprecise for leukopenic samples. We evaluated the efficacy of the Hematoflow method for determining the leukocyte differential in leukopenic samples and compared this method with the manual differential method.
METHODS
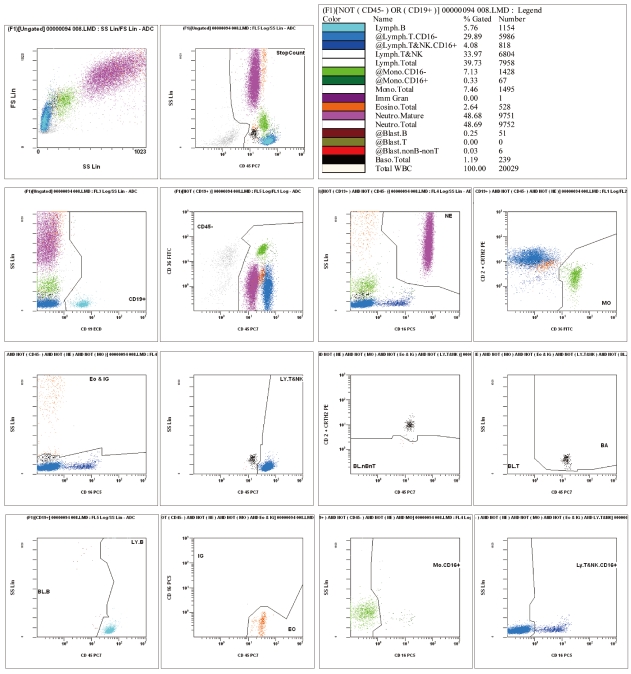
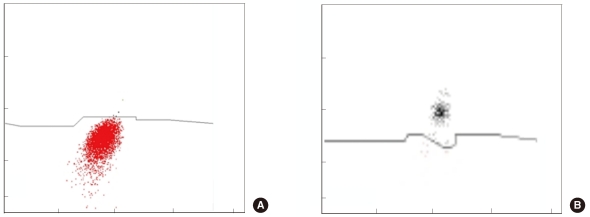
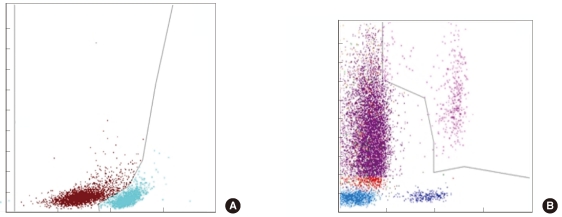
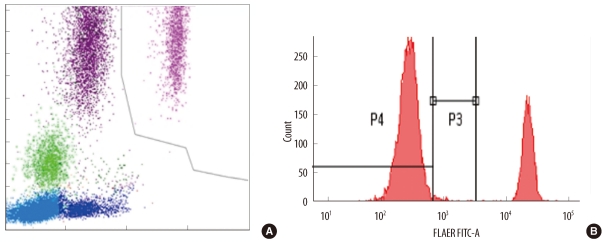
We selected 249 blood samples from 167 patients with leukopenia (WBC counts, 500-2,000/microL) for analysis in this study. The EDTA-anticoagulated blood samples were analyzed using an automatic blood cell counter (DxH800; Beckman Coulter, USA) and flow cytometry (FC 500; Beckman Coulter) by using Cytodiff reagent and analysis software (Beckman Coulter). Hematoflow results were selected or calculated from DxH800 and Cytodiff results. Two trained pathologists performed a manual differential count by counting 50-100 cells.
RESULTS
The precision of the Hematoflow method was superior to that of the manual method in counting 5 leukocyte subpopulations, immature granulocytes (IGs), and blasts. Blasts were detected in all 45 cases (100%) by Hematoflow. The correlation of the Cytodiff blast count to the reference count was high (r = 0.8325). For all other cell populations, the correlation of the Hematoflow results with the reference count was stronger than that of the other manual counts with the reference count.
CONCLUSIONS
The Hematoflow differential counting method is more reproducible and sensitive than manual counting, and is relatively easy to perform. In particular, this method detected leukemic blasts more sensitively than manual differential counts. The Hematoflow method is a very useful supplement to automated cell counting.
MeSH Terms
Figure
Cited by 2 articles
-
Flow Cytometric White Blood Cell Differential Using CytoDiff is Excellent for Counting Blasts
Jimin Kahng, Yonggoo Kim, Myungshin Kim, Eun-Jee Oh, Yeon-Joon Park, Kyungja Han
Ann Lab Med. 2015;35(1):28-34. doi: 10.3343/alm.2015.35.1.28.White blood cell differential counts in severely leukopenic samples: a comparative analysis of different solutions available in modern laboratory hematology
Ah Hyun Kim, Wonbae Lee, Myungshin Kim, Yonggoo Kim, Kyungja Han
Blood Res. 2014;49(2):120-126. doi: 10.5045/br.2014.49.2.120.
Reference
-
1. Guerti K, Vertessen F, Daniëls L, Van Der Planken M. Performance evaluation of the PENTRA 60C+ automated hematology analyzer and comparison with the ADVIA 2120. Int J Lab Hematol. 2009; 31:132–141. PMID: 19267810.2. Novis DA, Walsh M, Wilkinson D, St Louis M, Ben-Ezra J. Laboratory productivity and the rate of manual peripheral blood smear review: a college of American pathologists Q-probes study of 95,141 complete blood count determinations performed in 263 institutions. Arch Pathol Lab Med. 2006; 130:596–601. PMID: 16683868.
Article3. Barnes PW, McFadden SL, Machin SJ, Simson E. The international consensus group for hematology review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol. 2005; 11:83–90. PMID: 16024331.
Article4. Fuentes-Arderiu X, García-Panyella M, Dot-Bach D. Between-examiner reproducibility in manual differential leukocyte counting. Accred Qual Assur. 2007; 12:653–645.
Article5. Aapro M, Crawford J, Kamioner D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: where are we now? Support Care Cancer. 2010; 18:529–541. PMID: 20191292.
Article6. Mac Manus M, Lamborn K, Khan W, Varghese A, Graef L, Knox S. Radiotherapy-associated neutropenia and thrombocytopenia: analysis of risk factors and development of a predictive model. Blood. 1997; 89:2303–2310. PMID: 9116273.
Article7. Pierre RV. The demise of the eyecount leukocyte differential. Clin Lab Med. 2002; 22:279–297. PMID: 11933579.8. Cherian S, Levin G, Lo WY, Mauck M, Kuhn D, Lee C, et al. Evaluation of an 8-color flow cytometric reference method for white blood cell differential enumeration. Cytometry B Clin Cytom. 2010; 78:319–328. PMID: 20533390.
Article9. Björnsson S, Wahlström S, Norström E, Bernevi I, O'Neill U, Johansson E, et al. Total nucleated cell differential for blood and bone marrow using a single tube in a five-color flow cytometer. Cytometry B Clin Cytom. 2008; 74:91–103. PMID: 18061952.
Article10. Roussel M, Benard C, Ly-Sunnaram B, Fest T. Refining the white blood cell differential: the first flow cytometry routine application. Cytometry A. 2010; 77:552–563. PMID: 20506466.
Article11. Faucher JL, Lacronique-Gazaille C, Frébet E, Trimoreau F, Donnard M, Bordessoule D, et al. "6 Markers/5 Colors" Extended White Blood Cell Differential by Flow Cytometry. Cytometry A. 2007; 71:934–944. PMID: 17879238.
Article12. Shafer JA. Blood and marrow morphology in acute leukemia patients receiving chemotherapy: a photo-essay. Am J Med Technol. 1983; 49:77–90. PMID: 6573133.13. Jean A, Boutet C, Lenormand B, Callat MP, Buchonnet G, Barbay V, et al. The new haematology analyzer DxH 800: an evaluation of the analytical performances and leucocyte flags, comparison with the LH 755. Int J Lab Hematol. 2010; 8. 16. [Epub ahead of print].
Article14. Koenn ME, Kirby BA, Cook LL, Hare JL, Hall SH, Barry PM, et al. Comparison of four automated hematology cell analyzers. Clin Lab Sci. 2001; 14:238–242. PMID: 11760821.15. Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry. 1999; 38:139–152. PMID: 10440852.
Article16. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008; 112:1570–1580. PMID: 18725575.
Article17. Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006; 211:214–224. PMID: 16824130.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- White blood cell differential counts in severely leukopenic samples: a comparative analysis of different solutions available in modern laboratory hematology
- Laboratory Evaluation of the Coulter GEN.S System Five-Part Differential
- Evaluation of the Automated Hematology Analyzer Sysmex XN-2000 and the Accuracy of Differential Leukocyte Counts Using the Low WBC Mode
- Evaluation of Automated Flow Cytometric Analyzer Sysmex UF-100 for Cell Counting of Cerebrospinal Fluid
- Evaluation of the ABX Pentra DX 120 for the Detection of Immature Cells in Peripheral Blood