Korean J Radiol.
2008 Feb;9(1):45-53. 10.3348/kjr.2008.9.1.45.
Quantitative Assessment of Synovial Vascularity Using Contrast-Enhanced Power Doppler Ultrasonography: Correlation with Histologic Findings and MR Imaging Findings in Arthritic Rabbit Knee Model
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Radiology, Yonsei University College of Medicine, Seoul, Korea. jss@yumc.yonsei.ac.kr
- KMID: 1734274
- DOI: http://doi.org/10.3348/kjr.2008.9.1.45
Abstract
OBJECTIVE
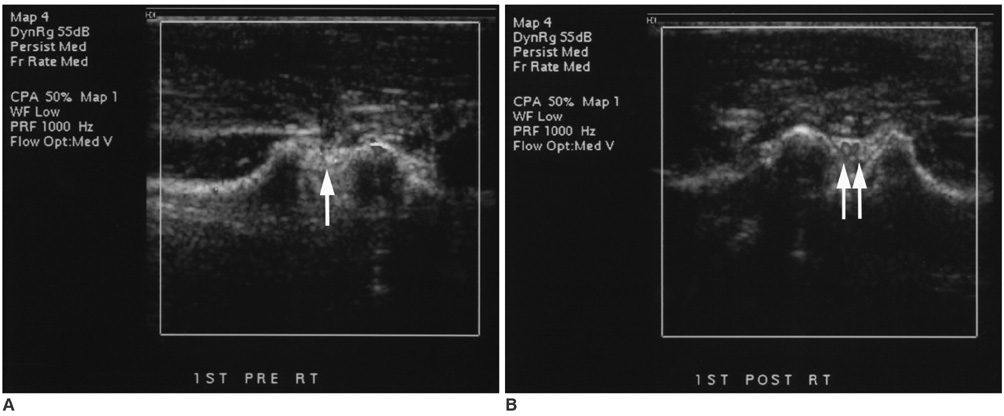
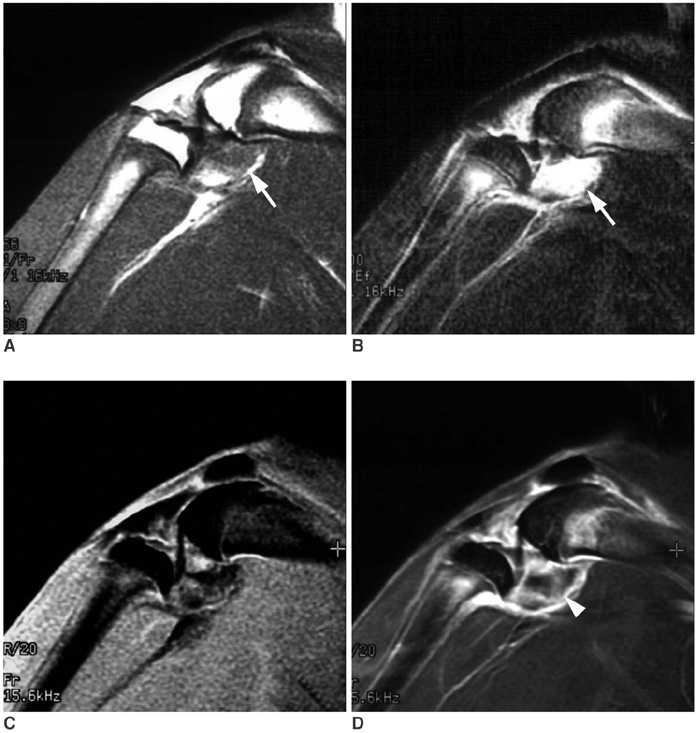
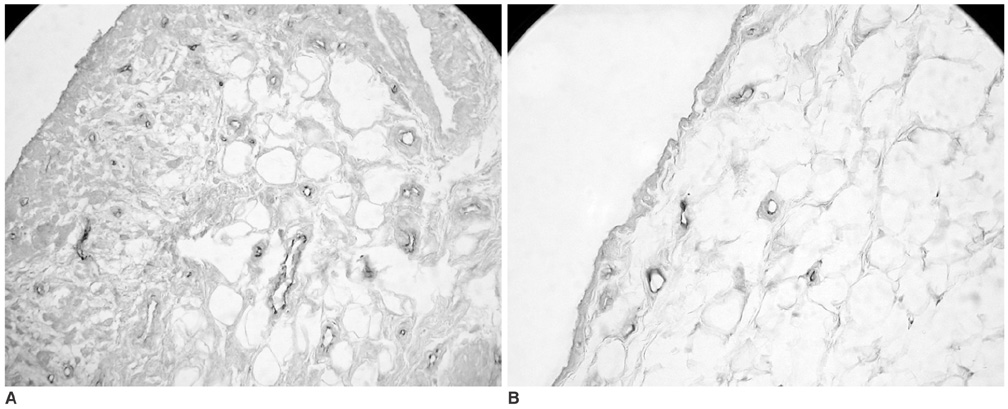
To validate contrast-enhanced power Doppler ultrasonography (PD US) for the evaluation of synovial vascularity in an arthritic rabbit knee model in correlation with MR and histological findings. MATERIALS AND METHODS: Power Doppler ultrasonography was performed for carrageenin-induced arthritic left knee and control right knee of 13 rabbits, first without and then with sonic contrast agent enhancement (Levovist, Schering, Berlin Germany), followed by gadolinium-enhanced MR imaging. Synovial vascularity was quantitatively assessed by calculating the color pixel area in power Doppler sonography using a computer-aided image analysis program and by grading the enhancement on MR images: grade 1, enhancement of knee joint is less than one-third of the area; grade 2, one-third to two-thirds enhancement; and grade 3, more than two-thirds enhancement. Microvessel density (MVD) was measured on slides stained immunohistochemically for CD31 antigen for histological assessment. RESULTS: The mean area of color pixels in PD US changed from 4.37 to 16.42 mm2 in the arthritic knee after enhancement (p < 0.05), whereas it changed from 0.77 to 2.31 mm2 in the control knee (p < 0.05). Arthritic knees had greater power Doppler signal than control knees both before and after contrast administration (p < 0.05). The average MVD was 88 in arthritic knees and 46 in control knees. MVDs correlated with color pixel areas of contrast-enhanced power Doppler imaging in arthritic knees. In MR grading of arthritic knees, five were grade 2 and eight were grade 3. MVD and PD US revealed no significant difference between grade 2 and 3 arthritic knees (p > 0.05). CONCLUSION: Sonic contrast-enhanced PD US improves the visualization of synovial vascularity and allows quantitative measurement in experimentally induced rabbit arthritic knees.
Keyword
MeSH Terms
-
Animals
Contrast Media
Gadolinium DTPA/diagnostic use
Image Processing, Computer-Assisted
Immunohistochemistry
Magnetic Resonance Imaging
Osteoarthritis, Knee/pathology/*ultrasonography
Polysaccharides/diagnostic use
Rabbits
Statistics, Nonparametric
Synovial Membrane/*blood supply/pathology/ultrasonography
*Ultrasonography, Doppler
Figure
Reference
-
1. Pando JA, Duray P, Yarboro C, Gourley MF, Klippel JH, Schumacher HR. Synovitis occurs in some clinically normal and asymptomatic joints in patients with early arthritis. J Rheumatol. 2000. 27:1848–1854.2. Harrison BJ, Symmons DP, Barrett EM, Silman AJ. The performance of the 1987 ARA classification criteria for rheumatoid arthritis in a population based cohort of patients with early inflammatory polyarthritis. American Rheumatism Association. J Rheumatol. 1998. 25:2324–2233.3. Schumacher HR, Pessler F, Chen LX. Diagnosing early rheumatoid arthritis (RA). What are the problems and opportunities? Clin Exp Rheumatol. 2003. 21:suppl. 31. S15–SS1.4. Saaibi DL, Schumacher HR Jr. Percutaneous needle biopsy and synovial histology. Baillieres Clin Rheumatol. 1996. 10:535–554.5. Ostergaard M, Szkudlarek M. Imaging in rheumatoid arthritis - why MRI and ultrasonography can no longer be ignored. Scand J Rheumatol. 2003. 32:63–73.6. Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Ostergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003. 48:955–962.7. Newman JS, Laing TJ, McCarthy CJ, Adler RS. Power Doppler sonography of synovitis: assessment of therapeutic response-preliminary observations. Radiology. 1996. 198:582–584.8. Walther M, Harms H, Krenn V, Radke S, Kirschner S, Gohlke F. Synovial tissue of the hip at power Doppler US: correlation between vascularity and power Doppler US signal. Radiology. 2002. 225:225–231.9. Carotti M, Salaffi F, Manganelli P, Salera D, Simonetti B, Grassi W. Power Doppler sonography in the assessment of synovial tissue of the knee joint in rheumatoid arthritis: a preliminary experience. Ann Rheum Dis. 2002. 61:877–882.10. Strouse PJ, DiPietro MA, Teo E-L, Doi K, Chrisp CE. Power Doppler evaluation of joint effusions: investigation in a rabbit model. Pediatr Radiol. 1999. 29:617–623.11. Wakefield RJ, Kong KO, Conaghan PG, Brown AK, O'Connor PJ, Emery P. The role of ultrasonography and magnetic resonance imaging in early rheumatoid arthritis. Clin Exp Rheumatol. 2003. 21(5 Suppl 31):S42–S49.12. Alasaarela E, Suramo I, Tervonen O, Lahde S, Takalo R, Hakala M. Evaluation of humeral head erosions in rheumatoid arthritis: a comparison of ultrasonography, magnetic resonance imaging, computed tomography and plain radiography. Br J Rheumatol. 1998. 37:1152–1156.13. Day SM, Lockhart JC, Ferrell WR, McLean JS. Divergent roles of nitrergic and prostanoid pathways in chronic joint inflammation. Ann Rheum Dis. 2004. 63:1564–1570.14. Liggins RT, Cruz T, Min W, Liang L, Hunter WL, Burt HM. Intra-articular treatment of arthritis with microsphere formulations of paclitaxel: biocompatibility and efficacy determinations in rabbits. Inflamm Res. 2004. 53:363–372.15. Hansra P, Moran EL, Fornasier VL, Bogoch ER. Carrageenan-induced arthritis in the rat. Inflammation. 2000. 24:141–155.16. van Dijke CF, Peterfy CG, Brasch RC, Lang P, Roberts TP, Shames D, et al. MR imaging of the arthritic rabbit knee joint using albumin-(Gd-DTPA)30 with correlation to histopathology. Magn Reson Imaging. 1999. 17:237–245.17. Katori M, Majima M, Harada Y. Possible background mechanisms of the effectiveness of cyclooxygenase-2 inhibitors in the treatment of rheumatoid arthritis. Inflamm Res. 1998. 47:Suppl 2. S107–S111.18. Gardner DL. Production of arthritis in the rabbit by the local injection of the mucopolysaccharide caragheenin. Ann Rheum Dis. 1960. 19:369–376.19. Uruchurtu Marroquin A, Ajmal M. Carrageenin-induced arthritis in the specific-pathogen-free pig. J Comp Pathol. 1970. 80:607–611.20. Di Rosa M. Biological properties of carrageenan. J Pharm Pharmacol. 1972. 24:89–102.21. Breedveld FC, Dijkmans BA. Differential therapy in early and late stages of rheumatoid arthritis. Curr Opin Rheumatol. 1996. 8:226–229.22. Brower AC. Use of the radiograph to measure the course of rheumatoid arthritis. The gold standard versus fool's gold. Arthritis Rheum. 1990. 33:316–324.23. Watt I. Basic differential diagnosis of arthritis. Eur Radiol. 1997. 7:344–351.24. Ostergaard M, Gideon P, Sorensen K, Hansen M, Stoltenberg M, Henriksen O, et al. Scoring of synovial membrane hypertrophy and bone erosions by MR imaging in clinically active and inactive rheumatoid arthritis of the wrist. Scand J Rheumatol. 1995. 24:212–218.25. Sharp JT. Radiologic assessment as an outcome measure in rheumatoid arthritis. Arthritis Rheum. 1989. 32:221–229.26. Kaye JJ. Arthritis: roles of radiography and other imaging techniques in evaluation. Radiology. 1990. 177:601–608.27. Resnick D. Resnick D, editor. Rheumatoid arthritis and the seronegative spondyloarthropathies: Radiographic and pathologic concepts. Diagnosis of bone and joint disorders. 2002. 4th ed. Philadelphia: Saunders;837–890.28. Scott DL. European preferences in assessing rheumatoid arthritis. J Rheumatol. 1993. 20:542.29. Felson DT. Choosing a core set of disease activity measures for rheumatoid arthritis clinical trials. J Rheumatol. 1993. 20:531–534.30. Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Sonne-Holm S, Lorenzen I. Quantification of synovistis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn Reson Imaging. 1998. 16:743–754.31. Gaffney K, Cookson J, Blades S, Coumbe A, Blake D. Quantitative assessment of the rheumatoid synovial microvascular bed by gadolinium-DTPA enhanced magnetic resonance imaging. Ann Rheum Dis. 1998. 57:152–157.32. Naranjo A, Marrero-Pulido T, Ojeda S, Francisco F, Erausquin C, Rua-Figueroa I, et al. Abnormal sonographic findings in the asymptomatic arthritic shoulder. Scand J Rheumatol. 2002. 31:17–21.33. Wakefield RJ, Gibbon WW, Conaghan PG, O'Connor P, McGonagle D, Pease C, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000. 43:2762–2770.34. Alarcon GS, Lopez-Ben R, Moreland LW. High-resolution ultrasound for the study of target joints in rheumatoid arthritis. Arthritis Rheum. 2002. 46:1969–1970. author reply 1970-1971.35. Firestein GS. Kelley WN, Ruddy S, Harris ED, Sledge CB, editors. Etiology and pathogenesis of rheumatoid arthritis. Textbook of rheumatology. 1997. 5th ed. Philadelphia: Saunders;851–897.36. Weber AJ, De Bandt M. Angiogenesis: general mechanisms and implications for rheumatoid arthritis. Joint Bone Spine. 2000. 67:366–383.37. Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001. 44:331–338.38. Magarelli N, Guglielmi G, Di Matteo L, Tartaro A, Mattei PA, Bonomo L. Diagnostic utility of an echo-contrast agent in patients with synovitis using power Doppler ultrasound: a preliminary study with comparison to contrast-enhanced MRI. Eur Radiol. 2001. 11:1039–1046.39. Klauser A, Frauscher F, Schirmer M, Halpern E, Pallwein L, Herold M, et al. The value of contrast-enhanced color Doppler ultrasound in the detection of vascularization of finger joints in patients with rheumatoid arthritis. Arthritis Rheum. 2002. 46:647–653.40. Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M. Contrast-enhanced power Doppler ultrasonography of the metacarpophalangeal joints in rheumatoid arthritis. Eur Radiol. 2003. 13:163–168.41. Fiocco U, Ferro F, Cozzi L, Vezzu M, Sfriso P, Checchetto C, et al. Contrast medium in power Doppler ultrasound for assessment of synovial vascularity: comparison with arthroscopy. J Rheumatol. 2003. 30:2170–2176.42. Klauser A, Frauscher F, Schirmer M. Value of contrast-enhanced power Doppler ultrasonography (US) of the metacarpophalangeal joints on rheumatoid arthritis. Eur Radiol. 2004. 14:545–546. author reply 547-548.43. Browne JE, Watson AJ, Hoskins PR, Elliott AT. Validation of a sensitivity performance index test protocol and evaluation of colour Doppler sensitivity for a range of ultrasound scanners. Ultrasound Med Biol. 2004. 30:1475–1483.44. Terslev L, Torp-Pedersen S, Savnik A, von der Recke P, Qvistgaard E, Danneskiold-Samsoe B, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: a comparative study. Arthritis Rheum. 2003. 48:2434–2441.45. Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M. Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001. 44:2018–2023.46. Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf KJ, et al. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999. 42:1232–1245.47. Wamser G, Bohndorf K, Vollert K, Bucklein W, Schalm J. Power Doppler sonography with and without echo-enhancing contrast agent and contrast-enhanced MRI for the evaluation of rheumatoid arthritis of the shoulder joint: differentiation between synovitis and joint effusion. Skeletal Radiol. 2003. 32:351–359.48. Wei K, Skyba DM, Firschke C, Jayaweera AR, Lindner JR, Kaul S. Interactions between microbubbles and ultrasound: in vitro and in vivo observations. J Am Coll Cardiol. 1997. 29:1081–1088.49. Albrecht T, Urbank A, Mahler M, Bauer A, Dore CJ, Blomley MJ, et al. Prolongation and optimization of Doppler enhancement with a microbubble US contrast agent by using continuous infusion: preliminary experience. Radiology. 1998. 207:339–347.50. Correas JM, Bridal L, Lesavre A, Mejean A, Claudon M, Helenon O. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol. 2001. 11:1316–1328.51. Schneider M. Bubbles and microcirculatory disorders. Eur Radiol. 2001. 11:Suppl 3. E1–E5.52. Immer FF, Seiler AM, Aeschbacher BC, Mahler F, Saner H. Influence of the ultrasound contrast agent Levovist on human nailfold capillary microcirculation. Angiology. 2000. 51:123–129.53. Turetschek K, Huber S, Floyd E, Helbich T, Roberts TP, Shames DM, et al. MR imaging characterization of microvessels in experimental breast tumors by using a particulate contrast agent with histopathologic correlation. Radiology. 2001. 218:562–569.54. Kim TJ, Moon WK, Cha JH, Goo JM, Lee KH, Kim KH, et al. VX2 carcinoma in rabbits after radiofrequency ablation: comparison of MR contrast agents for help in differentiating benign periablational enhancement from residual tumor. Radiology. 2005. 234:423–430.55. Turetschek K, Preda A, Novikov V, Brasch RC, Weinmann HJ, Wunderbaldinger P, et al. Tumor microvascular changes in antiangiogenic treatment: assessment by magnetic resonance contrast media of different molecular weights. J Magn Reson Imaging. 2004. 20:138–144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor Vascularity of Experimentally Induced VX2 Carcinoma in the Rabbit Thigh: Evaluation with Enhanced Power Doppler Sonography and DSA Correlated with Histopathologic Findings
- Up-to-date Doppler techniques for breast tumor vascularity: superb microvascular imaging and contrast-enhanced ultrasound
- Contrast-enhanced Harmonic Power Doppler Ultrasonography: Improved Depiction of Vascularity and Characterization of Flow Pattern in Hepatocellular Carcinoma
- MR Imaging of Multiple Fibroadenoma in Breast: Comparison with Color Doppler Images and Histologic Findings
- Intratumoral Vascularity of Experimentally Induced VX2 Carcinoma: Comparison of Power Doppler Sonography and Microangiography