J Korean Acad Periodontol.
2009 Jun;39(2):167-176. 10.5051/jkape.2009.39.2.167.
Effect of poly(lactide-co-glycolide) (PLGA) on bone regeneration in rabbit calvaria
- Affiliations
-
- 1Department of Periodontology, School of Dentistry, Wonkwang University, Korea. periohs@wonkwang.ac.kr
- KMID: 1733734
- DOI: http://doi.org/10.5051/jkape.2009.39.2.167
Abstract
-
PURPOSE: The purpose of this study is to histologically and histomorphometrically evaluate the effect of PLGA on bone regeneration compared with bone graft material.
METHODS
The experimental study was conducted in 10 rabbits with 2 different healing periods of 2 and 4 weeks. Following surgical exposure of the calvarium, 4 circular bone defects with a diameter of 4.6mm were formed. Rabbits were divided into control group, test groups I, and II. 10 defects assigned to the test group I were grafted with Nu-oss and other 10 defects assigned to the test group II were grafted with PLGA. The rest of the defects were in the negative control group. At 2nd and 4th week after surgery, 10 rabbits were sacrificed through intracardiac perfusion and then specimens were obtained. Histological analysis was performed following staining with trichorme and transversal sectioning of the calvarial bone.
RESULTS
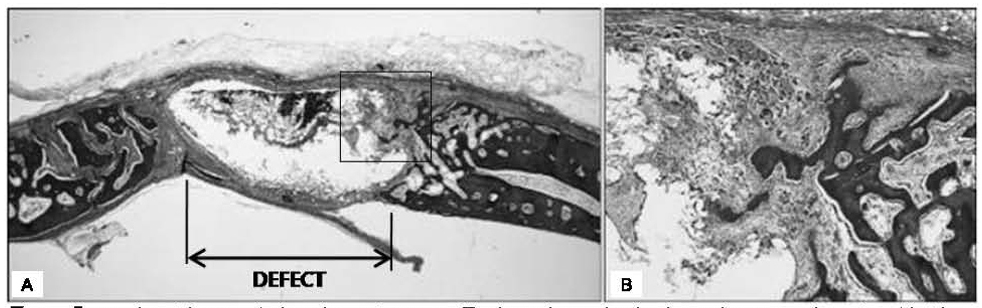
A group which used PLGA showed tissue reactions characterized by severe inflammation, rather than distinctive new bone formation.
CONCLUSIONS
The present experimental investigations have failed to prove any beneficial effects of PLGA. PLGA used in this study exhibited foreign body reactions and a less favorable pattern of new bone formation in comparison to control group.
CONCLUSION
PLGA did not function as scaffold. Further investigations of many types of micro PLGA that could improve its potential in GBR procedures are needed.
MeSH Terms
Figure
Reference
-
1. Urist MR, Dowell TA, Hay PH, Strates BS. Inductive substrates for bone formation. Clin Orthop Relat Res. 1968. 59:59–96.
Article2. Von Arx T, Broggini N, Jensen SS, et al. Membrane Durability and Tissue Response of Different Bioresorbable Barrier Membranes: A Histologic Study in the Rabbit Calvarium. INT J ORAL MAXILLOFAC IMPLANTS. 2005. 20:843–853.3. Hurzeler MB, Quinones CR, Hutmacher D, Schupbach P. Guided bone regeneration around dental implants in the atrophic alveolar ridge using a bioresorbable barrier. An experimental study in the monkey. Clin Oral Implants Res. 1997. 8:323–331.
Article4. Gher ME, Quintero G, Assad D, Monaco E, Richardson AC. Bone grafting and guided bone regeneration for immediate dental implants in humans. J Periodontol. 1994. 65:881–891.
Article5. Nowzari H, Slots J. Microbiologic and clinical study of polytetrafluoro-ethylene membranes for guided bone regeneration around implants. Int J Oral Maxillofac Implants. 1995. 10:67–73.6. Price RL, Waidb MC, Haberstroha KM, Webstera TJ. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials. 2003. 24:1877–1887.
Article7. Sakabe H, Ito H, Miyamoto T, Noishiki Y, Ha WS. In vivo blood compatibility of regenerated silk fibroin. Sen-i Gakkaish. 1989. 45:487–490.
Article8. Athreya SA, Martin DC. Impedance spectroscopy of protein polymer modified silicon micromachined probes. Sensors Actuators. 1999. 72:203–216.
Article9. Khil MS, Cha DI, Kim IS, Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res. 2003. 67B:675–679.
Article10. Bhattarai SR, Bhattarai N, Yi HK, et al. Novel biodegradable electrospun membrane: scaffold for tissue engineering. Biomaterials. 2004. 25:2595–2602.
Article11. Woodward SC, Brewer PS, Moatamed F. The intracellular degradation of poly(epsilon-caprolactone). J Biomed Mater Res. 1985. 19:437–444.12. Hutmacher DW, Garcia AJ. Scaffold-based bone engineering by using genetically modified cells. Gene. 2005. 347:1–10.
Article13. Yohko G, Masuhiro T, Norihiko M. Effect of the chemical modification of the arginyl residue in Bombyx mori silk fibroin on the attachment and growth of fibroblasts cells. J Biomed Mater Res. 1998. 39:351–357.14. Yohko G, Masuhiro T, Norihiko M, Yohji I. Synthesis of poly(ethylene glycol)-silk fibroin conjugates and surface in teraction between L-929 cells and the conjugates. Biomaterials. 1997. 18:267–271.
Article15. Minoura N, Aiba S, Gotoh Y, Tsukada M, Imai Y. Attachment and growth of cultured fibroblast cells on silk protein matrices. J Biomed Mater Res. 1995. 29:1215–1221.
Article16. Cai K, Yao K, Cui Y, et al. Influence of different surface modification treatments on poly(d,l-lactic acid) with silk fibroin and their effects on the culture of osteoblast in vitro. Biomaterials. 2002. 23:1603–1611.
Article17. Cai K, Yao K, Lin S, et al. Poly(d,l-lactic acid) surfaces modified by silk fibroin: effects on the culture of osteoblast in vitro. Biomaterials. 2002. 23:1153–1160.
Article18. Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997. 276:1425–1428.
Article19. van Kooten TG, Whitesides JF, von Recum AF. Influence of silicone (PDMS) surface texture on human skin fibroblast proliferation as determined by cell cycle analysis. J Biomed Mater Res. 1998. 43:1–14.
Article20. Min BM, Jeong L, Nam YS, et al. Formation of silk fibroin matrices with different texture and its cellular response to normal human keratinocytes. Int J Biol Macromol. 2004. 34:223–230.
Article21. Kim KH, Jeong L, Park HN, et al. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J Biotechnology. 2005. 120:327–339.
Article22. Becker W, Becker BE, Berg L, et al. New attachment after treatment with root isolation procedures; report for treated class III and class II furcations and vertical osseous defects. Int J Periodontics Restorative Dent. 1988. 8:8–23.23. Hutmacher DW, Kirsch A, Ackermann KL, Hürzeler MB. A tissue engineered cell-occlusive device for hard tissue regeneration-A preliminary report. Int J Periodontics Restorative Dent. 2001. 21:49–59.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation on the bone regenerative capacity of hyaluronic acid applied poly (D,L-lactic-co-glycolic acid) membranes in rabbit calvarial defect
- The sustaining effect of three polymers on the release of chlorhexidine from a controlled release drug device for root canal disinfection
- Encapsulation of Low Metronidazole Dose in Poly (D,L-lactide-co-glycolide) (PLGA) Nanoparticles Improves Giardia intestinalis Treatment
- Cellular responses on anodized titanium discs coated with 1 alpha,25-dihydroxyvitamin D3 incorporated Poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles
- The long-term study on the guided tissue regeneration with poly(alpha-hydroxy acid) membranes in beagle dogs