J Korean Med Sci.
2004 Oct;19(5):656-661. 10.3346/jkms.2004.19.5.656.
Genistein Supplementation Inhibits Atherosclerosis with Stabilization of the Lesions in Hypercholesterolemic Rabbits
- Affiliations
-
- 1Department of Pathology, Chungnam National University College of Medicine, Daejeon, Korea. cslee@cnu.ac.kr
- 2Department of Chest Surgery, Chungnam National University College of Medicine, Daejeon, Korea.
- 3Department of Food Service Industry Cheonan College of Foreign Studies, Cheonan, Korea.
- KMID: 1733504
- DOI: http://doi.org/10.3346/jkms.2004.19.5.656
Abstract
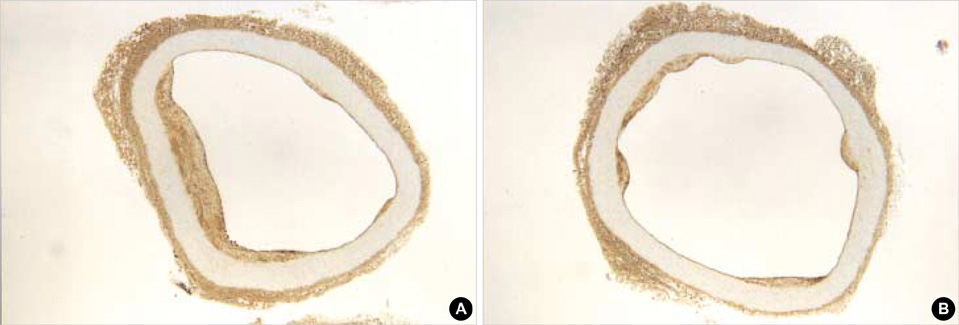
- The effect of genistein on aortic atherosclerosis was studied by immunohistochemistry with RAM-11 and HHF-35 antibodies and western blotting for matrix metalloproteinase-3 (MMP-3) in New Zealand White rabbits. After provocation of atherosclerosis with hyperlipidemic diet, the rabbits were divided as hyperlipidemic diet group (HD), normal diet group (ND) and hyperlipidemic plus genistein diet group (HD+genistein) for 4 and half months. The average cross sectional area of atherosclerotic lesion was 0.269 mm2 after provocation. The lesion was progressed by continuous hyperlipidemic diet (10.06 mm2) but was increased mildly by genistein (0.997 mm2), and decreased by normal diet (0.228 mm2). The ratio of macrophages to smooth muscle cells in the lesion was not changed by genistein supplementation. The western blotting showed reduction of MMP-3 expression in HD+genistein and ND groups than HD group. The inhibition of atherogenesis by genistein was might be due to improve the endothelial dysfunction rather than direct action on macrophages and/or smooth muscle cells in the lesion, since endothelial dysfunction by lipid peroxidation was the main atherogenic factor in the hypercholesterolemicrabbits. The genistein supplementation also suggests that it helps the stabilization of the atherosclerotic lesion by inhibition of MMP-3 expression.
Keyword
MeSH Terms
-
Animals
Aorta/pathology
Arteriosclerosis/*drug therapy/pathology/*prevention & control
Blotting, Western
Diet, Atherogenic
Genistein/*pharmacology
Growth Inhibitors/*pharmacology
Hypercholesterolemia/*drug therapy/pathology
Macrophages/pathology
Male
Muscle, Smooth, Vascular/enzymology/pathology
Rabbits
Research Support, Non-U.S. Gov't
Stromelysin 1/metabolism
Figure
Reference
-
1. Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest Suppl. 1990. 201:3–23.
Article2. Wong WW, Smith EO, Stuff JE, Hachey DL, Heird WC, Powell HJ. Cholesterol-lowering effect of soy protein in nomocholesterolemic and hypercholesterolemic men. Am J Clin Nutr. 1988. 68:1385S–1389S.3. Lichtenstein A. Soy protein, isoflavones and cardiovascular disease risk. J Nutr. 1998. 128:1589–1592.
Article4. Lee BS, Won JW, Lee SK, Choi Y, Yoon S, Park KH, Cho DJ, Song CH. The effect of isoflavone on serum lipid profiles and bone markers in postmenopausal woman. J Korean Soc Meno. 2002. 8:59–67.5. Dubey RK, Gillespie DG, Imthurn B, Rosselii M, Jackson E, Keller P. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension. 1999. 33:177–182.
Article6. Nestel PJ, Pomeroy SE, Sasahara T, Yamashita T, Liang YL, Dart AM, Jennings GL, Abbey M, Cameron JD. Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flasxseed oil despite increased LDL oxidizability. Arterioscler Thromb Vasc Biol. 1997. 17:1163–1170.7. Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17β-estradiol. Circulation. 2001. 103:258–262.
Article8. Murkies A. Phytoestrogens-what is the current knowledge? Australian Family Physician. 1998. 27:Suppl 1. S47–S51.9. Yamakoshi J, Piskula MK, Izumi T, Tobe K, Saito M, Kataoka S, Obata A, Kikuchi M. Isoflavone aglycone-rich extract without soy protein attenuates atherosclerosis development in cholesterol-fed rabbits. J Nutr. 2000. 130:1887–1893.
Article10. Libby P, Aikawa M. New insights into plaque stabilization by lipid lowering. Drugs. 1998. 56:Suppl. 9–13.11. Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989. 135:169–175.12. Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991. 11:1223–1230.
Article13. Hansson GK, Jonasson L, Holm J, Clowes MM, Clowes AW. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and vitro. Circ Res. 1988. 63:712–719.14. Galis ZS, Sukhova GK, Libby P. Microscopic localization of active proteases by situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995. 9:974–980.15. Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques: potentioal role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995. 92:1565–1569.16. Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinkerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998. 97:2433–2444.17. Shiomi M, Koh T, Tsudaka T. Reduction of serum cholesterol levels alters lesional composition of atherosclerotic plaques: effect of pravastatin sodium on atherosclerotis in mature WHHL rabbits. Arterioscl Thromb Vasc Biol. 1995. 15:1938–1944.18. Carson FL, Coleman SA, Futch HN. Hrapchak BB, Sheehan DC, editors. Connective tissue and muscle fiber stains. Theory and practice of histotechnology. 1987. Columbus, HO: Battelle Press;196–197.19. Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, Ohman L, Greene Gl, Gustafsson JA, Carlquiest M. Molecular basis of agonism and atntagonism in the oestrogen receptor. Nature. 1997. 389:753–758.20. Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990. 81:1680–1687.
Article21. Gilligan DM, Quyyumi AA, Cannon RO III. Effects of physiological levels of estrogen on coronary vasomotor function in post-menopausal women. Circulation. 1994. 89:2545–2551.
Article22. Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P. Estrogen improves endothelium-dependent, flow mediated vasodilation in postmenopausal women. Ann Intern Med. 1994. 121:936–941.23. Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, Deodato B, Ferlito M, Campo GM, Bova A, Caputi AP. Genistein supplementmentation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovascular Research. 2000. 45:454–462.24. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993. 362:801–809.
Article25. Ross R. Mechanism of disease: Atherosclerosis - An inflammatory diseasee. N Engl J Med. 1999. 340:115–126.26. Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic. coronary arteries of postmenopausal women. Circulation. 1994. 89:1501–1510.27. Stahl S, Chun T, Gray WG. Phytoestrogens act as estrogen agonists in an estrogen-responsive pituitary cell line. Toxicol Appl Pharmacol. 1998. 152:41–48.
Article28. van der Schouw YT, Pijpe A, Lebrun CE, Bots ML, Peeters PH, van Staveren WA, Lamberts SW, Grobbee DE. Higher usual dietary intake of phytoestrogens is associated with lower aortic stiffness in postmenopausal women. Arterioscler Thromb Vasc Biol. 2002. 22:1316–1322.
Article29. Honore EK, Williams JK, Anthony MS, Clarkson TB. Soy isoflavones enhance coronary vascular reactivity in atherosclerotic female macaques. Fertil Steril. 1997. 67:148–154.30. Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness A placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol. 2003. 23:1066–1071.31. Haft JI, Haik BJ, Goldstein JE, Brodyn NE. Development of significant coronary artery lesions in areas of minimal disease: a common mechanism for coronary disease progression. Chest. 1988. 94:731–736.32. Hackett D, Davies G, Maseri A. Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur Heart J. 1988. 9:1317–1323.
Article33. Mann JM, Davies MJ. Vulnerable Plaque. Relation of characteristics to degree of stenosis in human coronary arteries. Circulation. 1996. 94:928–931.34. Rioufol G, Finet G, Ginon I, André-Fouët X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF, Tabib A. Multiple Atherosclerotic Plaque Rupture in Acute Coronary Syndrome. Circulation. 2002. 106:804–808.
Article35. Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma. Circulation. 1998. 97:2433–2444.
Article36. Bocan TM, Krause BR, Rosebury WS, Mueller SB, Lu X, Dagle C, Major R, Lathia C, Lee H. The ACAT inhibitor Avasimibe reduces macrophages and matrix metalloprotenase expression in atherosclerotic lesions of hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 2000. 20:70–79.37. Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994. 94:2493–2503.
Article38. Nikkari ST, O'Brien KD, Ferguson M, Hatsukami T, Welgus HG, Alpers CE, Clowes AW. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 1995. 92:1393–1398.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dietary Fat and Genistein on Lipid Metabolism and Antioxidant Activity in Hyperlipidemic Male Rats induced High Fat Diet
- Experimental hypercholesterolemia induces ultrastructural changes in the elastic laminae of rabbit aortic valve
- Effects of Calcium and Genistein on Body Fat and Lipid Metabolism in High Fat-induced Obese Mice
- Anti-hypercholesterolemic and anti-atherosclerotic effects of polarized-light therapy in rabbits fed a high-cholesterol diet
- Involvement of ATPase in Impaired Endothelium-Dependent Relaxation of Rabbit corpus Cavernosum Smooth Muscle Induced by Hypercholesterolemia