Clin Exp Vaccine Res.
2014 Jul;3(2):235-243. 10.7774/cevr.2014.3.2.235.
DNA immunization of Mycobacterium tuberculosis resuscitation-promoting factor B elicits polyfunctional CD8+ T cell responses
- Affiliations
-
- 1Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, Daejeon, Korea. ecshin@kaist.ac.kr
- 2Department of Microbiology and Institute of Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1730629
- DOI: http://doi.org/10.7774/cevr.2014.3.2.235
Abstract
- PURPOSE
T cell-mediated immune responses, and particularly activation of polyfunctional T cells that simultaneously produce multiple cytokines, are necessary for the control of Mycobacterium tuberculosis. In the present study, we examined if DNA immunization of Mycobacterium tuberculosis resuscitation-promoting factor B (RpfB) elicits polyfunctional T cell responses in mice.
MATERIALS AND METHODS
C57BL/6 mice were immunized intramuscularly three times, at 3-week intervals, with RpfB-expressing plasmid DNA. For comparison, protein immunization was performed with recombinant RpfB in control mice. After immunization, RpfB-specific T cell responses were assessed by interferon-gamma (IFN-gamma) enzyme-linked immunosorbent spot assay and intracellular cytokine staining (ICS), and T cell polyfunctionality was assessed from the ICS data.
RESULTS
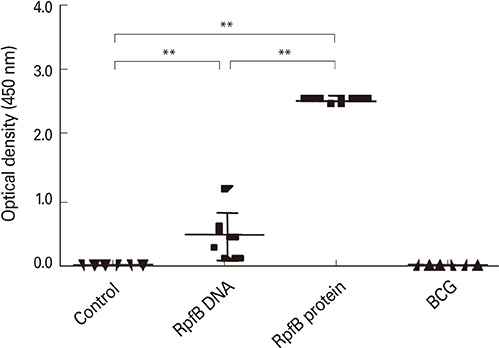
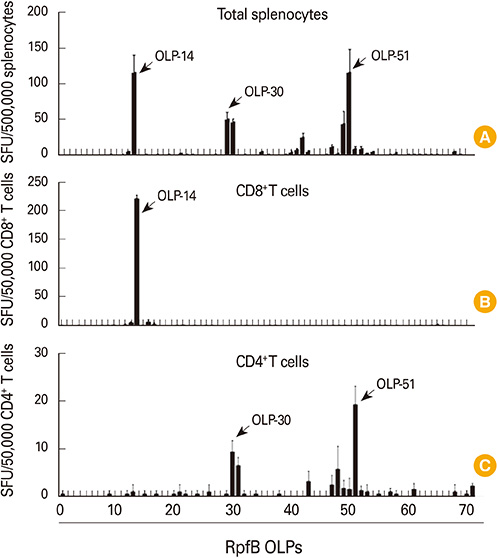
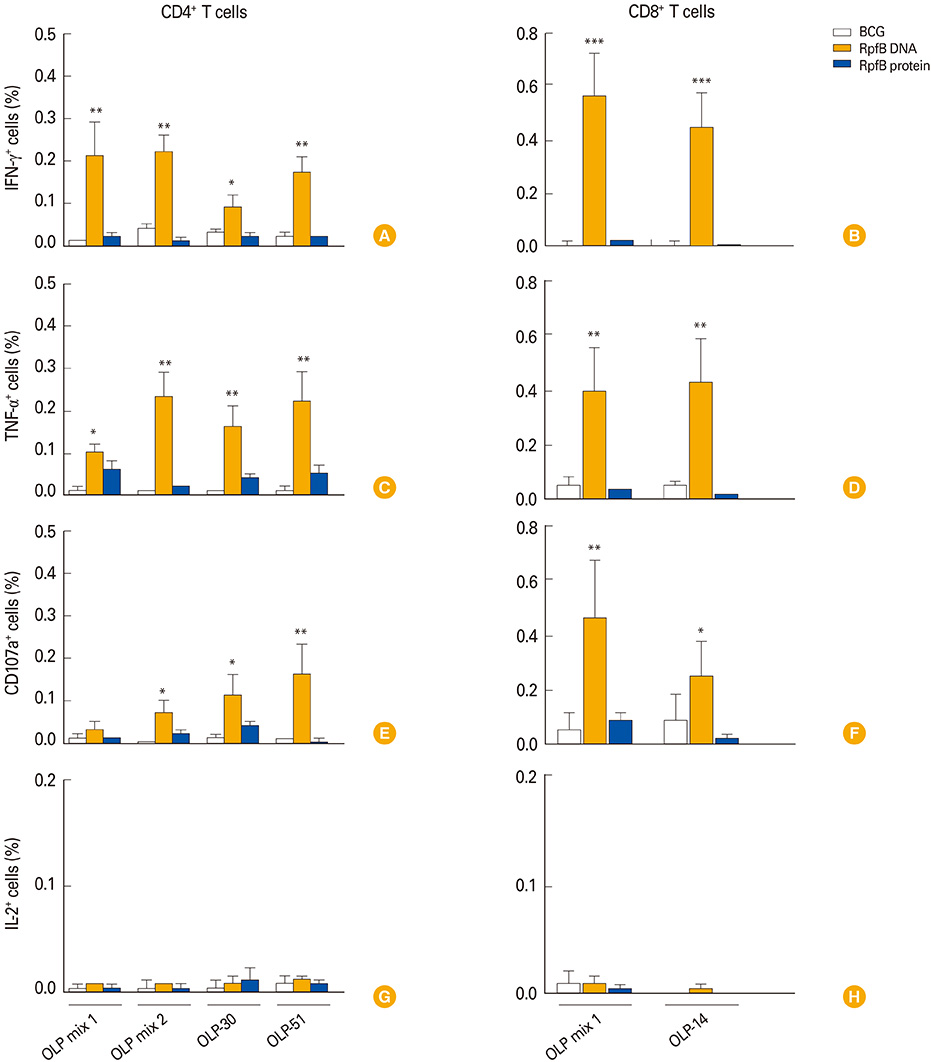
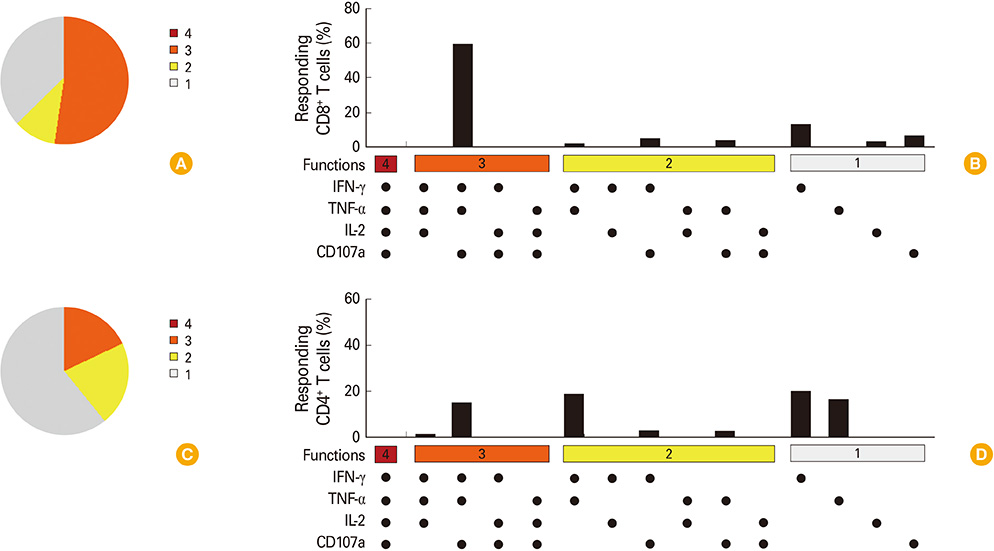
RpfB DNA immunization induced not only humoral immune responses, but also CD8+ and CD4+ T cell responses. Immunodominant T-cell epitopes were identified within RpfB by assays with overlapping peptides. RpfB DNA immunization elicited a polyfunctional CD8+ T cell response that was dominated by a functional phenotype of IFN-gamma+/TNF-alpha+/IL-2-/CD107a+.
CONCLUSION
RpfB DNA immunization elicits polyfunctional CD8+ T cell responses, suggesting that RpfB DNA immunization might induce protective immunity against tuberculosis.
MeSH Terms
Figure
Reference
-
1. Ottenhoff TH. Overcoming the global crisis: "yes, we can", but also for TB...? Eur J Immunol. 2009; 39:2014–2020.2. Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012; 54:784–791.
Article3. Behr MA, Wilson MA, Gill WP, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999; 284:1520–1523.
Article4. Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med. 1931; 24:1481–1490.
Article5. Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006; 367:1173–1180.
Article6. Colditz GA, Berkey CS, Mosteller F, et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995; 96(1 Pt 1):29–35.
Article7. Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994; 271:698–702.
Article8. Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995; 346:1339–1345.
Article9. Leveton C, Barnass S, Champion B, et al. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect Immun. 1989; 57:390–395.
Article10. Muller I, Cobbold SP, Waldmann H, Kaufmann SH. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987; 55:2037–2041.
Article11. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993; 178:2249–2254.
Article12. Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993; 178:2243–2247.
Article13. Chackerian AA, Perera TV, Behar SM. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect Immun. 2001; 69:2666–2674.
Article14. Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995; 2:561–572.
Article15. Lazarevic V, Flynn J. CD8+ T cells in tuberculosis. Am J Respir Crit Care Med. 2002; 166:1116–1121.16. Woodworth JS, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit Rev Immunol. 2006; 26:317–352.
Article17. Winau F, Weber S, Sad S, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006; 24:105–117.
Article18. Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007; 13:843–850.
Article19. Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008; 181:4955–4964.
Article20. Derrick SC, Yabe IM, Yang A, Morris SL. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine. 2011; 29:2902–2909.
Article21. Caccamo N, Meraviglia S, La Mendola C, Guggino G, Dieli F, Salerno A. Phenotypical and functional analysis of memory and effector human CD8 T cells specific for mycobacterial antigens. J Immunol. 2006; 177:1780–1785.
Article22. Caccamo N, Guggino G, Meraviglia S, et al. Analysis of Mycobacterium tuberculosis-specific CD8 T-cells in patients with active tuberculosis and in individuals with latent infection. PLoS One. 2009; 4:e5528.
Article23. Yeremeev VV, Kondratieva TK, Rubakova EI, et al. Proteins of the Rpf family: immune cell reactivity and vaccination efficacy against tuberculosis in mice. Infect Immun. 2003; 71:4789–4794.
Article24. Romano M, Aryan E, Korf H, et al. Potential of Mycobacterium tuberculosis resuscitation-promoting factors as antigens in novel tuberculosis sub-unit vaccines. Microbes Infect. 2012; 14:86–95.
Article25. Mukamolova GV, Kaprelyants AS, Young DI, Young M, Kell DB. A bacterial cytokine. Proc Natl Acad Sci U S A. 1998; 95:8916–8921.
Article26. Zhu W, Plikaytis BB, Shinnick TM. Resuscitation factors from mycobacteria: homologs of Micrococcus luteus proteins. Tuberculosis (Edinb). 2003; 83:261–269.
Article27. Downing KJ, Mischenko VV, Shleeva MO, et al. Mutants of Mycobacterium tuberculosis lacking three of the five rpf-like genes are defective for growth in vivo and for resuscitation in vitro. Infect Immun. 2005; 73:3038–3043.
Article28. Kana BD, Gordhan BG, Downing KJ, et al. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol Microbiol. 2008; 67:672–684.
Article29. Tufariello JM, Mi K, Xu J, et al. Deletion of the Mycobacterium tuberculosis resuscitation-promoting factor Rv1009 gene results in delayed reactivation from chronic tuberculosis. Infect Immun. 2006; 74:2985–2995.
Article30. Lee DH, Kim SH, Kang W, et al. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine. 2011; 29:8293–8301.
Article31. Choi YS, Lee JE, Nam SJ, et al. Two distinct functional patterns of hepatitis C virus (HCV)-specific T cell responses in seronegative, aviremic patients. PLoS One. 2013; 8:e62319.
Article32. Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006; 107:4781–4789.
Article33. Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007; 204:1405–1416.
Article34. Park SH, Shin EC, Capone S, et al. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology. 2012; 143:1048–1060.e4.
Article35. Smaill F, Jeyanathan M, Smieja M, et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med. 2013; 5:205ra134.
Article36. Abel B, Tameris M, Mansoor N, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med. 2010; 181:1407–1417.
Article37. Scriba TJ, Tameris M, Mansoor N, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol. 2010; 40:279–290.
Article38. Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008; 26:440–448.
Article39. Low L, Mander A, McCann K, et al. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009; 20:1269–1278.
Article40. Luckay A, Sidhu MK, Kjeken R, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007; 81:5257–5269.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Study of MHC class I Restricted CD8+ T Cell Mediated Immune Responses against Mycobacterium tuberculosis Infection: Evidence of M. tuberculosis Specific CD8+ T Cells in TB Patients and PPD+ Healthy Individuals
- A Case of Tuberculosis Verrucosa Cutis Confirmed by Detection of Mycobacterium tuberculosis DNA Using by Polymerase Chain Reaction
- M. tuberculosis Somatic Antigen Specific CD8+T cell Responses in BCG-Vaccinated Subjects
- Primer directed amplification of mycobacterium tuberculosis DNA in clinical specimens I. primers and reaction conditions
- CD8+ T Cell-mediated Immunity Induced by Heterologous Prime-boost Vaccination Based on DNA Vaccine and Recombinant Vaccinia Virus Expressing Epitope