Clin Exp Vaccine Res.
2014 Jul;3(2):227-234. 10.7774/cevr.2014.3.2.227.
Lumazine synthase protein cage nanoparticles as antigen delivery nanoplatforms for dendritic cell-based vaccine development
- Affiliations
-
- 1School of Life Sciences, Ulsan National Institute of Science and Technology, Ulsan, Korea. doy@unist.ac.kr
- KMID: 1730628
- DOI: http://doi.org/10.7774/cevr.2014.3.2.227
Abstract
- PURPOSE
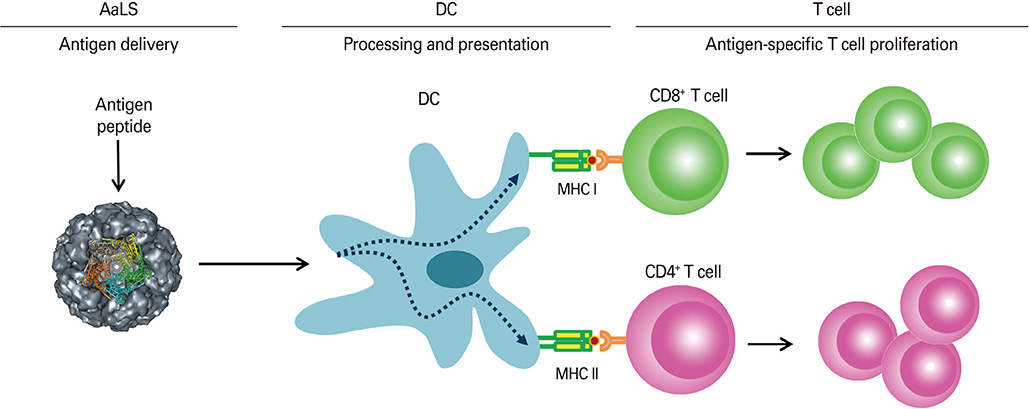
Protein cages are promising nanoplatform candidates for efficient delivery systems due to their homogenous size and structure with high biocompatibility and biodegradability. In this study, we investigate the potential of lumazine synthase protein cage as an antigen delivery system to dendritic cells (DCs), which induce antigen-specific T cell proliferation.
MATERIALS AND METHODS
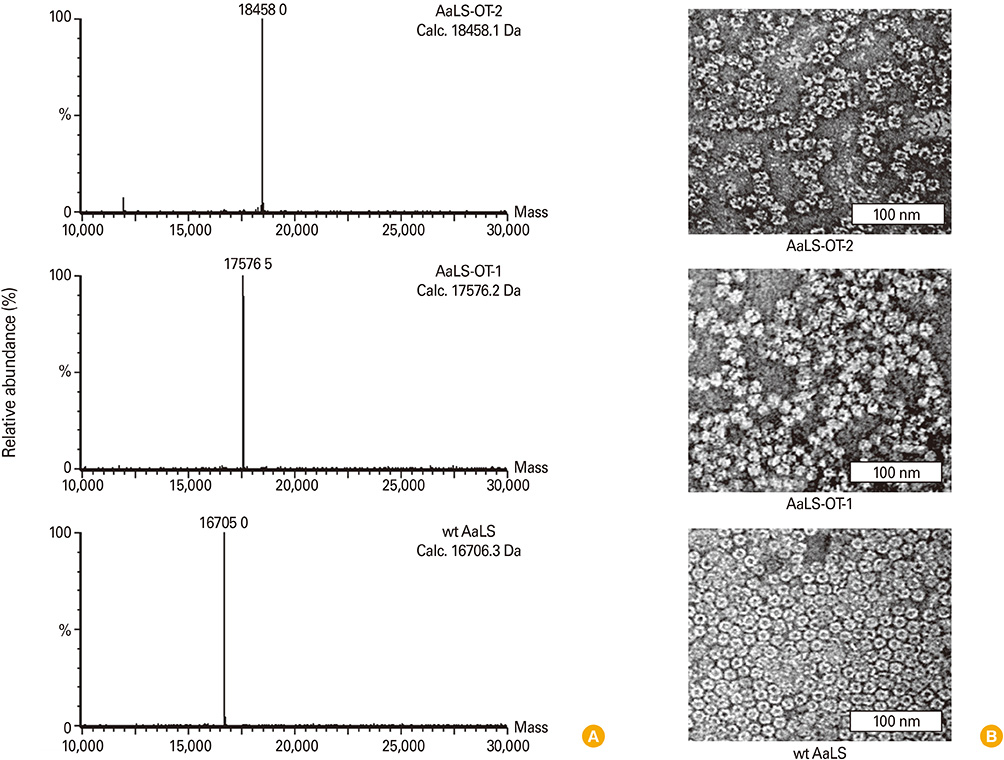
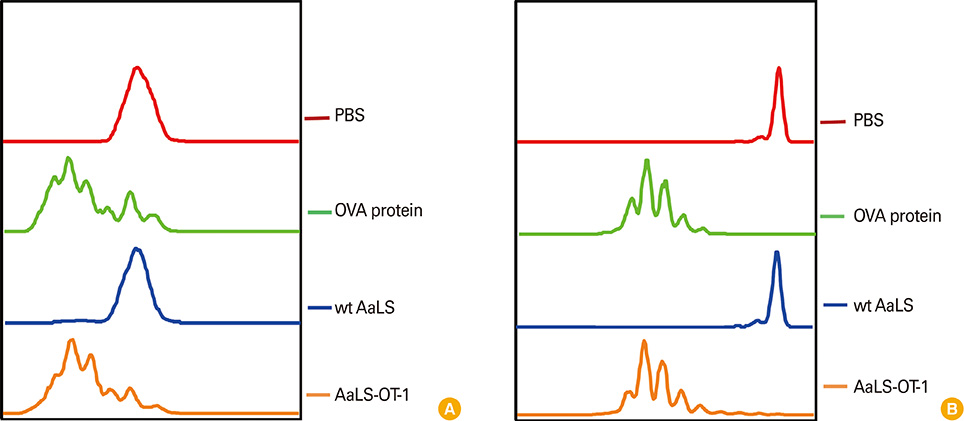
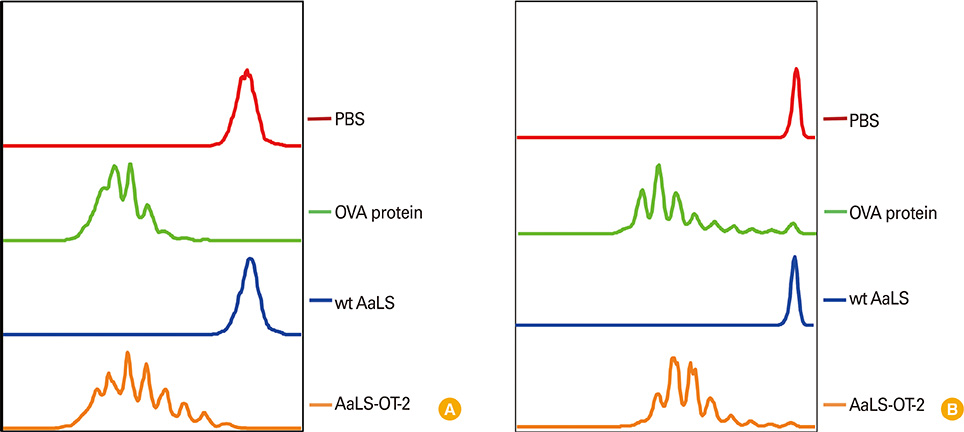
Ovalbumin (OVA) peptides OT-1 (SIINFEKL) and OT-2 (ISQAVHAAHAEINEAGR) were genetically inserted to lumazine synthase and each protein cage was over-expressed in Escherichia coli as a soluble protein. The efficiency of antigen delivery and the resulting antigen-specific T cell proliferation by DCs was examined in vitro as well as in vivo.
RESULTS
We successfully generated and characterized OVA peptides carrying lumazine synthase protein cages. The OT-1 and OT-2 peptides carried by lumazine synthases were efficiently delivered and processed by DCs in vitro as well as in vivo, and induced proliferation of OT-1-specific CD8+T cells and OT-2-specific CD4+T cells.
CONCLUSION
Our data demonstrate the potential of lumazine synthase protein cage being used as a novel antigen delivery system for DC-based vaccine development in future clinical applications.
MeSH Terms
Figure
Reference
-
1. Kang HJ, Kang YJ, Lee YM, Shin HH, Chung SJ, Kang S. Developing an antibody-binding protein cage as a molecular recognition drug modular nanoplatform. Biomaterials. 2012; 33:5423–5430.
Article2. Uchida M, Klem MT, Allen M, et al. Biological containers: protein cages as multifunctional nanoplatforms. Adv Mater. 2007; 19:1025–1042.
Article3. Kang S, Uchida M, O'Neil A, Li R, Prevelige PE, Douglas T. Implementation of p22 viral capsids as nanoplatforms. Biomacromolecules. 2010; 11:2804–2809.
Article4. Min J, Jung H, Shin HH, Cho G, Cho H, Kang S. Implementation of p22 viral capsids as intravascular magnetic resonance T1 contrast conjugates via site-selective attachment of Gd(III)-chelating agents. Biomacromolecules. 2013; 14:2332–2339.
Article5. Kang YJ, Park DC, Shin HH, Park J, Kang S. Incorporation of thrombin cleavage peptide into a protein cage for constructing a protease-responsive multifunctional delivery nanoplatform. Biomacromolecules. 2012; 13:4057–4064.
Article6. Uchida M, Kang S, Reichhardt C, Harlen K, Douglas T. The ferritin superfamily: supramolecular templates for materials synthesis. Biochim Biophys Acta. 2010; 1800:834–845.
Article7. Zhang X, Meining W, Fischer M, Bacher A, Ladenstein R. X-ray structure analysis and crystallographic refinement of lumazine synthase from the hyperthermophile Aquifex aeolicus at 1.6 A resolution: determinants of thermostability revealed from structural comparisons. J Mol Biol. 2001; 306:1099–1114.
Article8. Worsdorfer B, Pianowski Z, Hilvert D. Efficient in vitro encapsulation of protein cargo by an engineered protein container. J Am Chem Soc. 2012; 134:909–911.
Article9. Worsdorfer B, Woycechowsky KJ, Hilvert D. Directed evolution of a protein container. Science. 2011; 331:589–592.
Article10. Shenton W, Mann S, Colfen H, Bacher A, Fischer M. Synthesis of Nanophase Iron Oxide in Lumazine Synthase Capsids This work was supported by the BBSRC (W.S.). We thank A. M. Seddon for help with transmission electron microscopy and analytical ultracentrifufation studies and G. D. Ruggiero for the generation of computer images. Angew Chem Int Ed Engl. 2001; 40:442–445.11. Moon H, Kim WG, Lim S, et al. Fabrication of uniform layer-by-layer assemblies with complementary protein cage nanobuilding blocks via simple His-tag/metal recognition. J Mater Chem B. 2013; 1:4504–4510.
Article12. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012; 30:1–22.
Article13. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007; 449:419–426.
Article14. Han JA, Kang YJ, Shin C, et al. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomedicine. 2014; 10:561–569.
Article15. Trumpfheller C, Longhi MP, Caskey M, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. 2012; 271:183–192.
Article16. Do Y, Koh H, Park CG, et al. Targeting of LcrV virulence protein from Yersinia pestis to dendritic cells protects mice against pneumonic plague. Eur J Immunol. 2010; 40:2791–2796.
Article17. Flynn BJ, Kastenmuller K, Wille-Reece U, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A. 2011; 108:7131–7136.
Article18. Wang B, Zaidi N, He LZ, et al. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012; 14:R39.
Article19. Trumpfheller C, Finke JS, Lopez CB, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006; 203:607–617.
Article20. Wright A, Morrison SL. Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol. 1997; 15:26–32.
Article21. Lampkin BC, Levine AS, Levy H, Krivit W, Hammond D. Phase II trial of a complex polyriboinosinic-polyribocytidylic acid with poly-L-lysine and carboxymethyl cellulose in the treatment of children with acute leukemia and neuroblastoma: a report from the Children's Cancer Study Group. Cancer Res. 1985; 45(11 Pt 2):5904–5909.22. Batista-Duharte A, Lindblad EB, Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicol Lett. 2011; 203:97–105.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vaccine strategies utilizing C-type lectin receptors on dendritic cells in vivo
- Cancer Vaccines
- Distinct features of dendritic cell-based immunotherapy as cancer vaccines
- Immunogenicity of glycine nanoparticles containing a chimeric antigen as Brucella vaccine candidate
- Antigen Delivery Systems: Past, Present, and Future