Clin Exp Vaccine Res.
2014 Jul;3(2):155-167. 10.7774/cevr.2014.3.2.155.
Host immune responses to mycobacterial antigens and their implications for the development of a vaccine to control tuberculosis
- Affiliations
-
- 1Department of Microbiology and Infection Signaling Network Research Center, Chungnam National University School of Medicine, Daejeon, Korea. hayoungj@cnu.ac.kr
- KMID: 1730620
- DOI: http://doi.org/10.7774/cevr.2014.3.2.155
Abstract
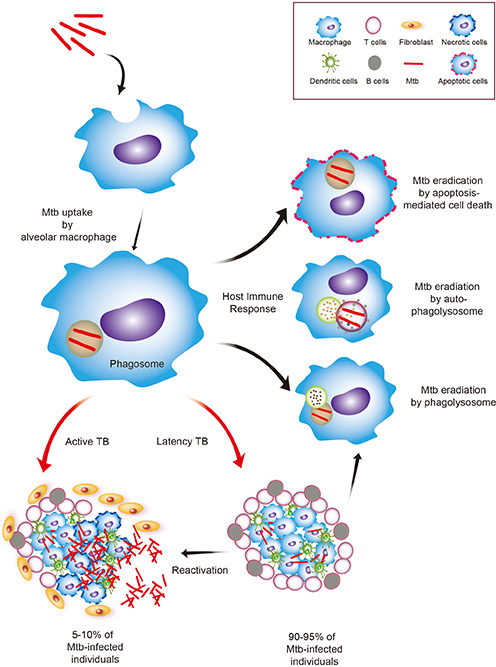
- Tuberculosis (TB) remains a worldwide health problem, causing around 2 million deaths per year. Despite the bacillus Calmette Guerin vaccine being available for more than 80 years, it has limited effectiveness in preventing TB, with inconsistent results in trials. This highlights the urgent need to develop an improved TB vaccine, based on a better understanding of host-pathogen interactions and immune responses during mycobacterial infection. Recent studies have revealed a potential role for autophagy, an intracellular homeostatic process, in vaccine development against TB, through enhanced immune activation. This review attempts to understand the host innate immune responses induced by a variety of protein antigens from Mycobacterium tuberculosis, and to identify future vaccine candidates against TB. We focus on recent advances in vaccine development strategies, through identification of new TB antigens using a variety of innovative tools. A new understanding of the host-pathogen relationship, and the usefulness of mycobacterial antigens as novel vaccine candidates, will contribute to the design of the next generation of vaccines, and to improving the host protective immune responses while limiting immunopathology during M. tuberculosis infection.
MeSH Terms
Figure
Cited by 1 articles
-
Mitogen-activated Protein Kinase Signaling in Inflammation-related Carcinogenesis
Zahid Manzoor, Jung Eun Koo, Young-Sang Koh
J Bacteriol Virol. 2014;44(4):297-304. doi: 10.4167/jbv.2014.44.4.297.
Reference
-
1. Kim HJ. Current status of tuberculosis in Korea. Korean J Med. 2012; 82:257–262.
Article2. Abubakar I, Zignol M, Falzon D, et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013; 13:529–539.
Article3. Kaufmann SH, Gengenbacher M. Recombinant live vaccine candidates against tuberculosis. Curr Opin Biotechnol. 2012; 23:900–907.
Article4. Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993; 22:1154–1158.
Article5. Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006; 367:1173–1180.
Article6. Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis.: meta-analysis of the published literature. JAMA. 1994; 271:698–702.
Article7. O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013; 31:475–527.8. Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994; 263:678–681.
Article9. Dietrich J, Doherty TM. Interaction of Mycobacterium tuberculosis with the host: consequences for vaccine development. APMIS. 2009; 117:440–457.
Article10. Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001; 19:93–129.
Article11. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993; 178:2249–2254.
Article12. Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995; 2:561–572.
Article13. Trumpfheller C, Longhi MP, Caskey M, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. 2012; 271:183–192.
Article14. Gannage M, Munz C. Autophagy in MHC class II presentation of endogenous antigens. Curr Top Microbiol Immunol. 2009; 335:123–140.15. Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009; 5:527–549.
Article16. Deretic V, Singh S, Master S, et al. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell Microbiol. 2006; 8:719–727.
Article17. Jo EK, Shin DM, Choi AM. Autophagy: cellular defense to excessive inflammation. Microbes Infect. 2012; 14:119–125.
Article18. Schmid D, Munz C. Immune surveillance via self digestion. Autophagy. 2007; 3:133–135.
Article19. Shaler CR, Horvath C, Lai R, Xing Z. Understanding delayed T-cell priming, lung recruitment, and airway luminal T-cell responses in host defense against pulmonary tuberculosis. Clin Dev Immunol. 2012; 2012:628293.
Article20. Ottenhoff TH. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol. 2012; 20:419–428.
Article21. Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998; 393:537–544.
Article22. Camus JC, Pryor MJ, Medigue C, Cole ST. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002; 148(Pt 10):2967–2973.
Article23. Louise R, Skjot V, Agger EM, Andersen P. Antigen discovery and tuberculosis vaccine development in the post-genomic era. Scand J Infect Dis. 2001; 33:643–647.
Article24. Singh S, Saraav I, Sharma S. Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis. Vaccine. 2014; 32:712–716.
Article25. Kunnath-Velayudhan S, Porcelli SA. Recent advances in defining the immunoproteome of Mycobacterium tuberculosis. Front Immunol. 2013; 4:335.
Article26. Houben EN, Nguyen L, Pieters J. Interaction of pathogenic mycobacteria with the host immune system. Curr Opin Microbiol. 2006; 9:76–85.
Article27. Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011; 4:288–293.
Article28. Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012; 12:581–591.
Article29. Shaler CR, Kugathasan K, McCormick S, et al. Pulmonary mycobacterial granuloma increased IL-10 production contributes to establishing a symbiotic host-microbe microenvironment. Am J Pathol. 2011; 178:1622–1634.30. Shaler CR, Horvath CN, Jeyanathan M, Xing Z. Within the Enemy's Camp: contribution of the granuloma to the dissemination, persistence and transmission of Mycobacterium tuberculosis. Front Immunol. 2013; 4:30.
Article31. Barry CE 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009; 7:845–855.
Article32. Gideon HP, Flynn JL. Latent tuberculosis: what the host "sees"? Immunol Res. 2011; 50:202–212.
Article33. Bertholet S, Ireton GC, Ordway DJ, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010; 2:53ra74.34. Lin MY, Ottenhoff TH. Not to wake a sleeping giant: new insights into host-pathogen interactions identify new targets for vaccination against latent Mycobacterium tuberculosis infection. Biol Chem. 2008; 389:497–511.
Article35. Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008; 21:279–286.
Article36. Hossain MM, Norazmi MN. Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection: the double-edged sword? Biomed Res Int. 2013; 2013:179174.37. Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb). 2010; 90:84–93.
Article38. Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004; 53:391–403.
Article39. Korf J, Stoltz A, Verschoor J, De Baetselier P, Grooten J. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen-associated host innate immune responses. Eur J Immunol. 2005; 35:890–900.
Article40. Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 2008; 10:995–1004.
Article41. Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006; 177:1805–1816.
Article42. Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000; 408:740–745.
Article43. Ferwerda G, Girardin SE, Kullberg BJ, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005; 1:279–285.
Article44. Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999; 285:732–736.
Article45. Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007; 9:1087–1098.
Article46. Jung SB, Yang CS, Lee JS, et al. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006; 74:2686–2696.
Article47. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011; 2011:405310.48. Davila S, Hibberd ML, Hari Dass R, et al. Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet. 2008; 4:e1000218.
Article49. Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005; 202:1715–1724.
Article50. Basu J, Shin DM, Jo EK. Mycobacterial signaling through toll-like receptors. Front Cell Infect Microbiol. 2012; 2:145.
Article51. Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998; 280:1432–1435.
Article52. de Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998; 280:1435–1438.
Article53. Fox CB, Moutaftsi M, Vergara J, et al. TLR4 ligand formulation causes distinct effects on antigen-specific cell-mediated and humoral immune responses. Vaccine. 2013; 31:5848–5855.
Article54. Orr MT, Fox CB, Baldwin SL, et al. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013; 172:190–200.
Article55. Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol. 2012; 188:2189–2197.
Article56. Desel C, Werninghaus K, Ritter M, et al. The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One. 2013; 8:e53531.
Article57. Kagina BM, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010; 182:1073–1079.
Article58. Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007; 8:369–377.
Article59. Gopal R, Rangel-Moreno J, Slight S, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013; 6:972–984.
Article60. Lindenstrom T, Woodworth J, Dietrich J, Aagaard C, Andersen P, Agger EM. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun. 2012; 80:3533–3544.
Article61. Gopal R, Lin Y, Obermajer N, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012; 42:364–373.
Article62. Cayabyab MJ, Macovei L, Campos-Neto A. Current and novel approaches to vaccine development against tuberculosis. Front Cell Infect Microbiol. 2012; 2:154.
Article63. Pitt JM, Stavropoulos E, Redford PS, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012; 189:4079–4087.
Article64. Zarate-Blades CR, Rodrigues RF, Souza PR, et al. Evaluation of the overall IFN-gamma and IL-17 pro-inflammatory responses after DNA therapy of tuberculosis. Hum Vaccin Immunother. 2013; 9:1093–1103.65. Agger EM, Cassidy JP, Brady J, Korsholm KS, Vingsbo-Lundberg C, Andersen P. Adjuvant modulation of the cytokine balance in Mycobacterium tuberculosis subunit vaccines; immunity, pathology and protection. Immunology. 2008; 124:175–185.
Article66. Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997; 65:623–629.
Article67. Ordway DJ, Costa L, Martins M, et al. Increased interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J Infect Dis. 2004; 190:756–766.
Article68. Glatman-Freedman A. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: advances toward a novel vaccine strategy. Tuberculosis (Edinb). 2006; 86:191–197.
Article69. Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol. 2009; 39:676–686.
Article70. Zhang F, Lu YJ, Malley R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc Natl Acad Sci U S A. 2013; 110:13564–13569.
Article71. Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004; 432:1032–1036.
Article72. Jo EK, Yuk JM, Shin DM, Sasakawa C. Roles of autophagy in elimination of intracellular bacterial pathogens. Front Immunol. 2013; 4:97.
Article73. Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004; 119:753–766.
Article74. Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009; 6:231–243.
Article75. Munz C. Enhancing immunity through autophagy. Annu Rev Immunol. 2009; 27:423–449.
Article76. Ravindran R, Khan N, Nakaya HI, et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science. 2014; 343:313–317.
Article77. Ni Cheallaigh C, Keane J, Lavelle EC, Hope JC, Harris J. Autophagy in the immune response to tuberculosis: clinical perspectives. Clin Exp Immunol. 2011; 164:291–300.
Article78. Tey SK, Khanna R. Host immune system strikes back: autophagy-mediated antigen presentation bypasses viral blockade of the classic MHC class I processing pathway. Autophagy. 2012; 8:1839–1841.79. Monath TP. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev Vaccines. 2012; 11:427–448.
Article80. Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009; 15:267–276.
Article81. Meerak J, Wanichwecharungruang SP, Palaga T. Enhancement of immune response to a DNA vaccine against Mycobacterium tuberculosis Ag85B by incorporation of an autophagy inducing system. Vaccine. 2013; 31:784–790.
Article82. Romagnoli A, Etna MP, Giacomini E, et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012; 8:1357–1370.
Article83. Content J, de la Cuvellerie A, De Wit L, Vincent-Levy-Frebault V, Ooms J, De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991; 59:3205–3212.
Article84. Wiker HG, Sletten K, Nagai S, Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990; 58:272–274.
Article85. Anderson DH, Harth G, Horwitz MA, Eisenberg D. An interfacial mechanism and a class of inhibitors inferred from two crystal structures of the Mycobacterium tuberculosis 30 kDa major secretory protein (Antigen 85B), a mycolyl transferase. J Mol Biol. 2001; 307:671–681.
Article86. Abou-Zeid C, Ratliff TL, Wiker HG, Harboe M, Bennedsen J, Rook GA. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988; 56:3046–3051.
Article87. Malen H, Softeland T, Wiker HG. Antigen analysis of Mycobacterium tuberculosis H37Rv culture filtrate proteins. Scand J Immunol. 2008; 67:245–252.
Article88. Kashyap RS, Ramteke SP, Deshpande PS, Purohit HJ, Taori GM, Daginawala HF. Comparison of an adenosine deaminase assay with ELISA for the diagnosis of tuberculous meningitis infection. Med Sci Monit. 2007; 13:BR200–BR204.89. Horwitz MA, Lee BW, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1995; 92:1530–1534.
Article90. McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4(+) t-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect Immun. 2001; 69:681–686.
Article91. Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003; 171:1602–1609.
Article92. Williams A, Goonetilleke NP, McShane H, et al. Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infect Immun. 2005; 73:3814–3816.
Article93. Verreck FA, Vervenne RA, Kondova I, et al. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One. 2009; 4:e5264.
Article94. McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004; 10:1240–1244.
Article95. Sander CR, Pathan AA, Beveridge NE, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med. 2009; 179:724–733.
Article96. Pathan AA, Sander CR, Fletcher HA, et al. Boosting BCG with recombinant modified vaccinia ankara expressing antigen 85A: different boosting intervals and implications for efficacy trials. PLoS One. 2007; 2:e1052.
Article97. Scriba TJ, Tameris M, Mansoor N, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol. 2010; 40:279–290.
Article98. Brookes RH, Hill PC, Owiafe PK, et al. Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa. PLoS One. 2008; 3:e2921.
Article99. Hawkridge T, Scriba TJ, Gelderbloem S, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis. 2008; 198:544–552.
Article100. Ibanga HB, Brookes RH, Hill PC, et al. Early clinical trials with a new tuberculosis vaccine, MVA85A, in tuberculosis-endemic countries: issues in study design. Lancet Infect Dis. 2006; 6:522–528.
Article101. Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013; 381:1021–1028.
Article102. Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001; 69:2773–2778.
Article103. McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci. 2011; 366:2782–2789.104. Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006; 177:6353–6360.
Article105. Kamath AT, Rochat AF, Valenti MP, et al. Adult-like anti-mycobacterial T cell and in vivo dendritic cell responses following neonatal immunization with Ag85B-ESAT-6 in the IC31 adjuvant. PLoS One. 2008; 3:e3683.
Article106. Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun. 2004; 72:6148–6150.
Article107. Agger EM, Rosenkrands I, Olsen AW, et al. Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine. 2006; 24:5452–5460.
Article108. van Dissel JT, Arend SM, Prins C, et al. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine. 2010; 28:3571–3581.
Article109. Wang S, Chen J, Zhang Y, et al. Mycobacterium tuberculosis region of difference (RD) 2 antigen Rv1985c and RD11 antigen Rv3425 have the promising potential to distinguish patients with active tuberculosis from M. bovis BCG-vaccinated individuals. Clin Vaccine Immunol. 2013; 20:69–76.
Article110. Ansari MA, Zubair S, Mahmood A, et al. RD antigen based nanovaccine imparts long term protection by inducing memory response against experimental murine tuberculosis. PLoS One. 2011; 6:e22889.
Article111. Lindenstrom T, Agger EM, Korsholm KS, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009; 182:8047–8055.
Article112. Ganguly N, Siddiqui I, Sharma P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis (Edinb). 2008; 88:510–517.
Article113. Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002; 46:709–717.
Article114. Lewis KN, Liao R, Guinn KM, et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J Infect Dis. 2003; 187:117–123.
Article115. Aagaard C, Hoang T, Dietrich J, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011; 17:189–194.
Article116. Dietrich J, Aagaard C, Leah R, et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005; 174:6332–6339.
Article117. Luo Y, Wang B, Hu L, et al. Fusion protein Ag85B-MPT64 (190-198)-Mtb8.4 has higher immunogenicity than Ag85B with capacity to boost BCG-primed immunity against Mycobacterium tuberculosis in mice. Vaccine. 2009; 27:6179–6185.
Article118. Majlessi L, Rojas MJ, Brodin P, Leclerc C. CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infect Immun. 2003; 71:7173–7177.
Article119. Kashino SS, Pollock N, Napolitano DR, Rodrigues V Jr, Campos-Neto A. Identification and characterization of Mycobacterium tuberculosis antigens in urine of patients with active pulmonary tuberculosis: an innovative and alternative approach of antigen discovery of useful microbial molecules. Clin Exp Immunol. 2008; 153:56–62.
Article120. Mukherjee S, Kashino SS, Zhang Y, et al. Cloning of the gene encoding a protective Mycobacterium tuberculosis secreted protein detected in vivo during the initial phases of the infectious process. J Immunol. 2005; 175:5298–5305.
Article121. Napolitano DR, Pollock N, Kashino SS, Rodrigues V Jr, Campos-Neto A. Identification of Mycobacterium tuberculosis ornithine carboamyltransferase in urine as a possible molecular marker of active pulmonary tuberculosis. Clin Vaccine Immunol. 2008; 15:638–643.
Article122. Cayabyab MJ, Kashino SS, Campos-Neto A. Robust immune response elicited by a novel and unique Mycobacterium tuberculosis protein using an optimized DNA/protein heterologous prime/boost protocol. Immunology. 2012; 135:216–225.
Article123. Mukai T, Tsukamoto Y, Maeda Y, Tamura T, Makino M. Efficient activation of human T cells of both CD4 and CD8 subsets by urease-deficient recombinant Mycobacterium bovis BCG that produced a heat shock protein 70-M. tuberculosis-derived major membrane protein II fusion protein. Clin Vaccine Immunol. 2014; 21:1–11.
Article124. Kim JS, Kim WS, Choi HG, et al. Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. J Leukoc Biol. 2013; 94:733–749.
Article125. Byun EH, Kim WS, Kim JS, et al. Mycobacterium tuberculosis Rv0577, a novel TLR2 agonist, induces maturation of dendritic cells and drives Th1 immune response. FASEB J. 2012; 26:2695–2711.
Article126. Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011; 10:645–658.
Article127. Grode L, Ganoza CA, Brohm C, Weiner J 3rd, Eisele B, Kaufmann SH. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013; 31:1340–1348.
Article128. Perez E, Samper S, Bordas Y, Guilhot C, Gicquel B, Martin C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol Microbiol. 2001; 41:179–187.
Article129. Arbues A, Aguilo JI, Gonzalo-Asensio J, et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013; 31:4867–4873.
Article130. Hoft DF, Blazevic A, Abate G, et al. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis. 2008; 198:1491–1501.
Article131. Leroux-Roels I, Leroux-Roels G, Ofori-Anyinam O, et al. Evaluation of the safety and immunogenicity of two antigen concentrations of the Mtb72F/AS02(A) candidate tuberculosis vaccine in purified protein derivative-negative adults. Clin Vaccine Immunol. 2010; 17:1763–1771.
Article132. Leroux-Roels I, Forgus S, De Boever F, et al. Improved CD4(+) T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine. 2013; 31:2196–2206.
Article133. Sun R, Skeiky YA, Izzo A, et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009; 27:4412–4423.
Article134. Nicol MP, Grobler LA. MVA-85A, a novel candidate booster vaccine for the prevention of tuberculosis in children and adults. Curr Opin Mol Ther. 2010; 12:124–134.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunological Mechanisms by Which Concomitant Helminth Infections Predispose to the Development of Human Tuberculosis
- microRNAs in Mycobacterial Infection: Modulation of Host Immune Response and Apoptotic Pathways
- The Role of Nitric Oxide in Mycobacterial Infections
- The Roles of Chemokines in Immune Response to Mycobacterial Infection
- Host-Pathogen Dialogues in Autophagy, Apoptosis, and Necrosis during Mycobacterial Infection