J Korean Med Sci.
2008 Apr;23(2):176-184. 10.3346/jkms.2008.23.2.176.
Cost-Benefit Analysis of Haemophilus Influenzae Type B Immunization in Korea
- Affiliations
-
- 1Department of Health Care Management and Policy, Seoul National University, School of Public Health, Seoul, Korea.
- 2Department of Preventive Medicine, Hanyang University, College of Medicine, Seoul, Korea. yshin@hanyang.ac.kr
- 3Department of Preventive Medicine, Eulji University, School of Medicine, Daejeon, Korea.
- KMID: 1713446
- DOI: http://doi.org/10.3346/jkms.2008.23.2.176
Abstract
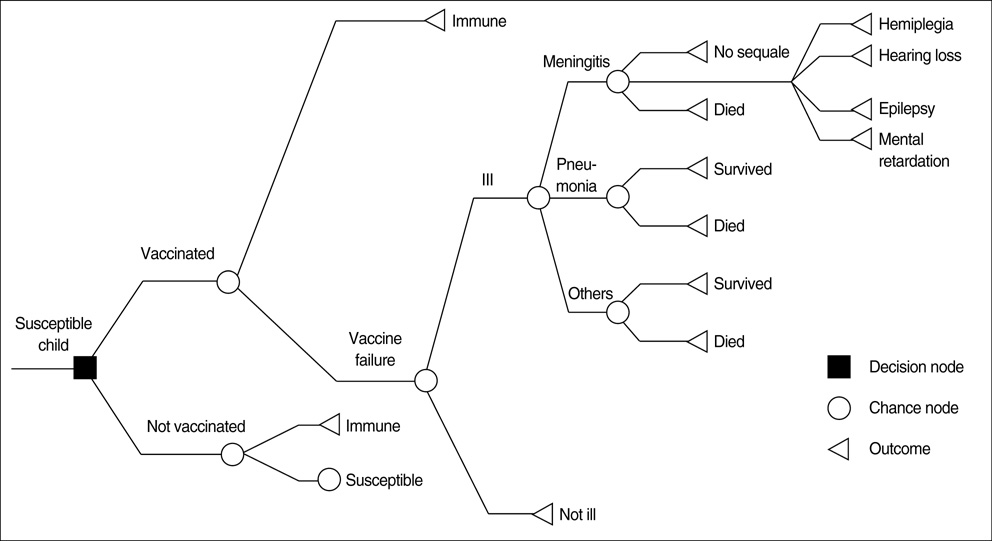
- An economic evaluation of Haemophilus influenzae type b (Hib) immunization was conducted to examine whether Hib immunization should be included in the Korea's national immunization program. The costs and benefits included direct and indirect values and an estimation of the economic efficiency. We determined that a universal Hib immunization program in Korea would prevent 17 deaths and 280 invasive Hib cases. When we assumed the one Hib immunization cost as 26,000 won, the national Hib immunization would cost 34.6 billion won. Costs for various Hib diseases were estimated at 26.8 billion won (11.8 billion won from direct costs and 14.9 billion won from indirect costs). A benefit-cost ratio of 0.77 showed that the economic efficiency of the integration of Hib immunization in Korea is low because of the low incidence rate of Hib disease and high price of vaccine. However, if the Hib immunization cost decrease to less than 20,000 won, a benefit-cost ratio increase to 1.0 and above, integrating Hib immunization into the national immunization program with economic efficiency can be considered.
MeSH Terms
-
Child
Child, Preschool
Cost of Illness
Cost-Benefit Analysis
Decision Support Techniques
Haemophilus Infections/*economics/*prevention & control
Haemophilus Vaccines/*economics/*therapeutic use
Haemophilus influenzae type b/*metabolism
Humans
Immunization/*economics
Immunization Schedule
Infant
Korea
Models, Economic
State Medicine
Figure
Cited by 2 articles
-
Is Hib Vaccine of Economic Value in South Korea?
Ulla K. Griffiths, Karen Edmond, Rana Hajjeh
J Korean Med Sci. 2009;24(1):187-187. doi: 10.3346/jkms.2009.24.1.187.Authors' Reply to "Is Hib Vaccine of Economic Value in South Korea?"
Sangjin Shin, Young-jeon Shin, Moran Ki
J Korean Med Sci. 2009;24(1):188-188. doi: 10.3346/jkms.2009.24.1.188.
Reference
-
1. WHO. Global Programme for Vaccines and Immunizations (GPV). The WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec. 1998. 73:64–68.2. Schillinger J, Schwartz B, Perkins B, Wenger J. Murray CJL, Lopez A, editors. Global burden of bacterial meningitis. The global burden of diseases. 1996. Boston: Harvard School of Public Health (on behalf of the World Health Organization and the World Bank).3. WHO. Introduction of Haemophilus influenzae type b vaccine into immunization programmes: Department of Vaccines and Biologicals. 2000. WHO.4. Levine OS, Schwartz B, Pierce N, Kane M. Development, evaluation and implementation of Haemophilus influenzae type b vaccines for young children in developing countries: current status and priority actions. Pediatr Infect Dis J. 1998. 17:9 Suppl. S95–S113.
Article5. Immunization. cited 2007. May 20. WHO Regional Office for the Western Pacific;Available from:URL: http://www.wpro.who.int/health_topics/immunization/general_info.htm.6. Hussain I, Syed A, Sofiah A, Ong L, Choo K, Musa M, Teh K, Ng H. Cost-Benefit analysis of Haemophilus influenzae vaccination programme in Malaysia. Buletin Kasiharan Masyakat Jilid. 1999. 5:79–89.7. Kim JS, Jang YT, Kim JD, Park TH, Park JM, Kilgore PE, Kennedy WA, Park E, Nyambat B, Kim DR, Hwang PH, Kim SJ, Eun SH, Lee HS, Cho JH, Kim YS, Chang SJ, Huang HF, Clemens JD, Ward JI. Incidence of Haemophilus influenzae type b and other invasive diseases in South Korean children. Vaccine. 2004. 22:3952–3962.
Article8. Shin E, Lee M, Kwon S, Ki M, Kim K, Na B, Nam H, Lee S. Development of vaccination coverage estimation methods and evaluation indicators of national immunization program in Korea (in Korean). 2005. SEOUL: Korea Center for Disease Control and Prevention.9. Zhou F, Bisgard K, Yusuf H, Deuson R, Bath S, Murphy T. Impact of universal Haemophilus influenzae type b vaccination starting at 2 months of age in the United States: an economic analysis. Pediatrics. 2002. 110:653–661.
Article10. Baraff L, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993. 12:389–394.11. Jiménez FJ, Guallar-Castillón P, Rubio Terrés C, Guallar E. Cost-benefit analysis of Haemophilus influenzae type b vaccination in children in Spain. Pharmacoeconomics. 1999. 15:75–83.
Article12. Dagan R, Fraser D, Roitman M, Slater P, Anis E, Ashkenazi S, Kassis I, Miron D, Leventhal A. Effectiveness of a nationwide infant immunization program against Haemophilus influenzae b. The Israeli Pediatric Bacteremia and Meningitis Group. Vaccine. 1999. 17:134–141.13. CDC. U.S. Public Health Service. A practical guide to prevention effectiveness: decision and economic analysis. 1994. Atlanta(GA):14. Jeong H. Public financing in total health expenditure and coverage by National Health Insurance in Korea: Including comparison with other OECD countries (in Korean). Korean J Health Economics Policy. 2003. 10:95–112.15. Drummond M, Sculpher M, Torrance G, O'Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. 2005. 3rd ed. New York: Oxford University Press.16. Ministry of Labor. Survey Report on Wage Structure (in Korean). 2005. Korea National Statistical Office.17. Korea Institute for Health & Social Affairs. Average age of parents at their first child birth(in Korean). 2005. Korea National Statistical Office.18. Zepp F, Schuind A, Meyer C, Sanger R, Kaufhold A, Willems P. Safety and reactogenicity of a novel DTPa-HBV-IPV combined vaccine given along with commercial Hib vaccines in comparison with separate concomitant administration of DTPa, Hib, and OPV vaccines in infants. Pediatrics. 2002. 109:e58.19. Livartowski A, Boucher J, Detournay B, Reinert P. Cost-effectiveness evaluation of vaccination against Haemophilus influenzae invasive diseases in France. Vaccine. 1996. 14:495–500.
Article20. Korea National Statistical Office. Annual report on the economically active population survey (in Korean). 2003. Korea National Statistical Office.21. Limcangco MR, Armour CL, Salole EG, Taylor SJ. Cost-benefit analysis of a Haemophilus influenzae type b meningitis prevention programme in The Philippines. Pharmacoeconomics. 2001. 19:391–400.
Article22. Trollfors B. Cost-benefit analysis of general vaccination against Haemophilus influenzae type b in Sweden. Scand J Infect Dis. 1994. 26:611–614.
Article23. Chen RT, Rastogi SC, Mullen JR, Hayes SW, Cochi SL, Donlon JA, Wassilak SG. The Vaccine Adverse Event Reporting System (VAERS). Vaccine. 1994. 12:542–550.
Article24. Choung E, Kim Y, Kim Y, Kim D, Seo J, Lee H. Immunogenicity and safety of a Haemophilus influenzae type b polysaccharide-tetanus toxiod conjugate vaccine(PRP-T; Hiberix) in Korean infants (in Korean). Korean J Pediat Infect Dis. 2003. 10:71–80.25. Korean Phamaceutical Traders Association. Import record situation of an integrative medicine item by item(in Korean). 2003. SEOUL: Korean Phamaceutical Traders Association.26. Peltola H. Haemophilus influenzae type b disease and vaccination in Europe: lessons learned. Pediatr Infect Dis J. 1998. 17:9 Suppl. S126–S132.
Article27. Lau YL, Yung R, Low L, Sung R, Leung CW, Lee WH. Haemophilus influenzae type b infections in Hong Kong. Pediatr Infect Dis J. 1998. 17:9 Suppl. S165–S169.
Article28. Yang Y, Shen X, Jiang Z, Liu X, Leng Z, Lu D, Rao J, Liu J, Chang L. Study on Haemophilus influenzae type b diseases in China: the past, present and future. Pediatr Infect Dis J. 1998. 17:9 Suppl. S159–S165.
Article29. Lee YS, Kumarasinghe G, Chow C, Khor E, Lee BW. Invasive Haemophilus influenzae type b infections in Singapore children: a hospital-based study. J Paediatr Child Health. 2000. 36:125–127.30. Kamiya H, Uehara S, Kato T, Shiraki K, Togashi T, Morishima T, Goto Y, Satoh O, Standaert SM. Childhood bacterial meningitis in Japan. Pediatr Infect Dis J. 1998. 17:9 Suppl. S183–S185.
Article31. Nakano T, Ihara T, Kamiya H, Yabu Y, Kuwabara H, Iwade Y, Sugiyama A. Incidence of Haemophilus influenzae type b meningitis in Mie prefecture, Japan. Pediatr Int. 2001. 43:323–324.
Article32. WHO. Report of a meeting on priorities for pneumococcal and Haemophilus influenzae type b(Hib) vaccine development and introduction. 2001. Geneva: Department of Vaccines and Biologicals, WHO.33. Goeree R, O'Brien B, Blackhouse G, Agro K, Goering P. The valuation of productivity costs due to premature mortality: a comparison of the human-capital and friction-cost methods for schizophrenia. Can J Psychiatry. 1999. 44:455–463.
Article34. Brinsmead R, Hill S, Walker D. Are economic evaluations of vaccines useful to decision-makers? Case study of Haemophilus influenzae type b vaccines. Pediatr Infect Dis J. 2004. 23:32–37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Present status and prospects of Haemophilus influenzae type b(Hib) immunization
- A Case of Neurologic Sequelae and a Case of Peripheral Gangrene of Extremities Associated with Haemophilus influenzae Type b Meningitis
- Natural Anti-PRP Antibody Levels ot Haemophilus Influenzae Type b(Hib) and Changs of Antibody Levels after Three Doses of Vacination
- Haemophilus influenzae vaccine
- Cerebral Venous Sinus Thrombosis with Meningitis and Septicemia due to Haemophilus influenzae Type f in an Immunocompetent Child