J Korean Med Sci.
2007 Oct;22(5):883-890. 10.3346/jkms.2007.22.5.883.
Microarray Analysis of Thyroid Stimulating Hormone, Insulin-Like Growth Factor-1, and Insulin-Induced Gene Expression in FRTL-5 Thyroid Cells
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. yjparkmd@snu.ac.kr
- 3Human Genome Research Institute, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- KMID: 1713298
- DOI: http://doi.org/10.3346/jkms.2007.22.5.883
Abstract
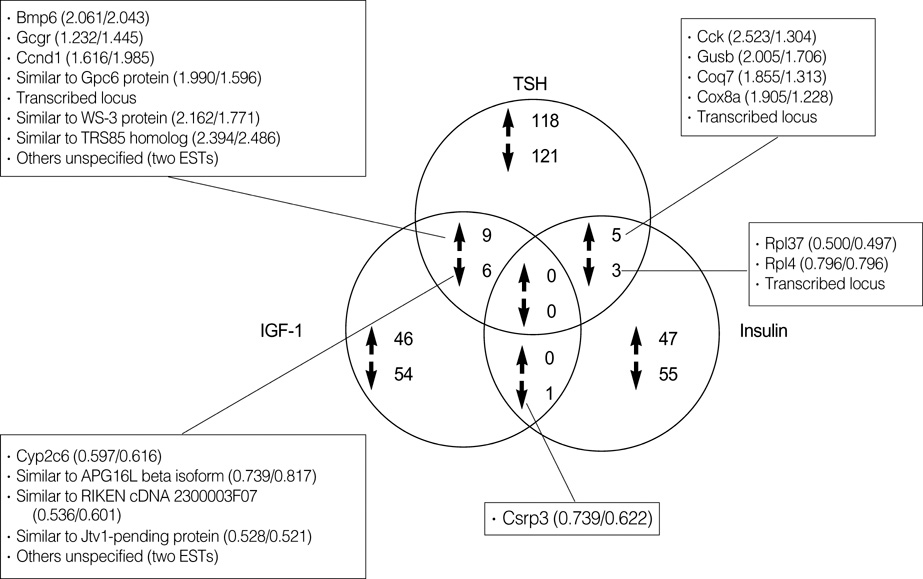
- To determine which genes are regulated by thyroid stimulating hormone (thyrotropin, TSH), insulin and insulin-like growth factor-1 (IGF-1) in the rat thyroid, we used the microarray technology and observed the changes in gene expression. The expressions of genes for bone morphogenetic protein 6, the glucagon receptor, and cyclin D1 were increased by both TSH and IGF-1; for cytochrome P450, 2c37, the expression was decreased by both. Genes for cholecystokinin, glucuronidase, beta, demethyl-Q 7, and cytochrome c oxidase, subunit VIIIa, were up-regulated; the genes for ribosomal protein L37 and ribosomal protein L4 were down-regulated by TSH and insulin. However, there was no gene observed to be regulated by all three: TSH, IGF-1, and insulin molecules studied. These findings suggest that TSH, IGF-1, and insulin stimulate different signal pathways, which can interact with one another to regulate the proliferation of thyrocytes, and thereby provide additional influence on the process of cellular proliferation.
Keyword
MeSH Terms
-
Animals
Bone Morphogenetic Protein 6
Bone Morphogenetic Proteins/biosynthesis
Cell Line, Tumor
Cyclin D1/biosynthesis
*Gene Expression Profiling
*Gene Expression Regulation
Insulin/*biosynthesis/metabolism
Insulin-Like Growth Factor I/*biosynthesis
Models, Genetic
*Oligonucleotide Array Sequence Analysis
Rats
Receptors, Glucagon/biosynthesis
Thyroid Gland/*metabolism
Thyrotropin/*biosynthesis/metabolism
Time Factors
Figure
Reference
-
1. Mathai V, Idikula J, Fenn AS, Nair A. Do long-standing nodular goitres result in malignancies? Aust N Z J Surg. 1994. 64:180–182.
Article2. Hishinuma A, Fukata S, Kakudo K, Murata Y, Ieiri T. High incidence of thyroid cancer in long-standing goiters with thyroglobulin mutations. Thyroid. 2005. 15:1079–1084.
Article3. Tramontano D, Moses AC, Veneziani BM, Ingbar SH. Adenosine 3', 5'-monophosphate mediates both the mitogenic effect of thyrotropin and its ability to amplify the response to insulin-like growth factor I in FRTL5 cells. Endocrinology. 1988. 122:127–132.4. Takahashi S, Conti M, Van Wyk JJ. Thyrotropin potentiation of insulin-like growth factor-I dependent deoxribonucleic acid synthesis in FRTL-5 cells: mediation by an autocrine amplification factor(s). Endocrinology. 1990. 126:736–745.
Article5. Yamamoto K, Hirai A, Ban T, Saito J, Tahara K, Terano T, Tamura Y, Saito Y, Kitagawa M. Thyrotropin induces G1 cyclin expression and accelerates G1 phase after insulin-like growth factor I stimulation in FRTL-5 cells. Endocrinology. 1996. 137:2036–2042.
Article6. Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. Regulation of thyroid cell proliferation by TSH and other factors?: a critical evaluation of in vitro models. Endocr Rev. 2001. 22:631–656.
Article7. Park YJ, Kim TY, Lee SH, Kim H, Kim SW, Shong M, Yoon YK, Cho BY, Park DJ. p66Shc expression in proliferating thyroid cells is regulated by thyrotropin receptor signaling. Endocrinology. 2005. 146:2473–2480.
Article8. Yamazaki K, Yamada E, Kanaji Y, Yanagisawa T, Kato Y, Takano K, Obara T, Sato K. Genes regulated by thyrotropin and iodide in cultured human thyroid follicles: analysis by cDNA microarray. Thyroid. 2003. 13:149–158.
Article9. Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002. 30:e15.
Article10. Deleu S, Savonet V, Behrends J, Dumont JE, Maenhaut C. Study of gene expression in thyrotropin-stimulated thyroid cells by cDNA expression array: ID3 transcription modulating factor as an early response protein and tumor marker in thyroid carcinoma. Exp Cell Res. 2002. 279:62–70.11. Bruno R, Ferretti E, Tosi E, Arturi F, Giannasio P, Mattei T, Scipioni A, Presta I, Morisi R, Gulino A, Filetti S, Russo D. Modulation of thyroid-specific gene expression in normal and nodular human thyroid tissues from adults: an in vivo effect of thyrotropin. J Clin Endocrinol Metab. 2005. 90:5692–5697.
Article12. Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999. 112:3519–3527.
Article13. Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998. 125:1005–1015.
Article14. Kraunz KS, Nelson HH, Liu M, Wiencke JK, Kelsey KT. Interaction between the bone morphogenetic proteins and Ras/MAP-kinase signalling pathways in lung cancer. Br J Cancer. 2005. 93:949–952.
Article15. Suzuki J, Otsuka F, Takeda M, Inagaki K, Miyoshi T, Mimura Y, Ogura T, Doihara H, Makino H. Functional roles of the bone morphogenetic protein system in thyrotropin signaling in porcine thyroid cells. Biochem Biophys Res Commun. 2005. 327:1124–1130.
Article16. Hansen LH, Abrahamsen N, Nishimura E. Glucagon receptor mRNA distribution in rat tissues. Peptides. 1995. 16:1163–1166.
Article17. Morales A, Lachuer J, Duchamp C, Vera N, Georges B, Cohen-Adad F, Moulin C, Barre H. Tissue-specific modulation of rat glucagon receptor mRNA by thyroid status. Mol Cell Endocrinol. 1998. 144:71–81.
Article18. Navarro I, Leibush B, Moon TW, Plisetskaya EM, Banos N, Mendez E, Planas JV, Gutierrez J. Insulin, insulin-like growth factor-I (IGF-I) and glucagon: the evolution of their receptors. Comp Biochem Physiol B Biochem Mol Biol. 1999. 122:137–153.
Article19. Ginda WJ. Evidence for a functional role of cholecystokinin receptors in the rat thyroid gland. Folia Histochem Cytobiol. 2001. 39:331–334.20. Ahren B. Regulatory peptides in the thyroid gland--a review on their localization and function. Acta Endocrinol (Copenh). 1991. 124:225–232.21. Timler D, Tazbir J, Matejkowska M, Gosek A, Czyz W, Brzezinski J. Expression of proteins: D1 cyclin and Ki-67 in papillary thyroid carcinomas. Folia Histochem Cytobiol. 2001. 39:Suppl 2. 201–202.22. Wang S, Lloyd RV, Hutzler MJ, Safran MS, Patwardhan NA, Khan A. The role of cell cycle regulatory protein, cyclin D1, in the progression of thyroid cancer. Mod Pathol. 2000. 13:882–887.
Article23. Lubitz CC, Gallagher LA, Finley DJ, Zhu B, Fahey TJ 3rd. Molecular analysis of minimally invasive folllicular carcinomas by gene profiling. Surgery. 2005. 138:1042–1048.24. Yoo HY, Oh SK, Yi KH, Cho BY, Koh CS. Effect of growth factors on the expression of proto-oncogenes c-fos and c-myc in FRTL-5 cell line. J Korean Med Sci. 1995. 10:155–163.
Article25. Takada K, Amino N, Tada H, Miyai K. Relationship between proliferation and cell cycle-dependent Ca2+ influx induced by a combination of thyrotropin and insulin-like growth factor-1 in rat thyroid cells. J Clin Invest. 1990. 86:1548–1555.26. Vandeput F, Zabeau M, Maenhaut C. Identification of differentially expressed genes in thyrotropin stimulated dog thyroid cells by the cDNA-AFLP technique. Mol Cell Endocrinol. 2005. 243:58–65.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hormonal and Cytokine Regulation of ICAM-1 gene in FRTL-5 Thyroid Cells: Cloning and Analysis of 5-Regulatory Region of Rat ICAM-1 Gene
- Hormonal regulation of ICAM-1 gene expression in thyroid cells, FRTL-5
- Cell density dependence of growth characteristics of rat thyroid cells(FRTL-5) stimulated by TSH and IGF-I
- Effect of ceramide on apoptosis and phospholipase D activity in FRTL-5 thyroid cells
- Effect of Lithium on Na+/I- Symporter Gene Expression in Rat Thyroid FRTL-5 Cells