J Korean Med Sci.
2005 Aug;20(4):636-642. 10.3346/jkms.2005.20.4.636.
Epstein-Barr Virus and p16INK4A Methylation in Squamous Cell Carcinoma and Precancerous Lesions of the Cervix Uteri
- Affiliations
-
- 1Department of Pathology, Gachon Medical School Gil Medical Center, Incheon, Korea.
- 2Department of Pathology, Guro Hospital, Korea University College of Medicine, Seoul, Korea. iskim@korea.ac.kr
- 3Department of Surgery, College of Medicine, Konkuk University, Chungju, Korea.
- KMID: 1712746
- DOI: http://doi.org/10.3346/jkms.2005.20.4.636
Abstract
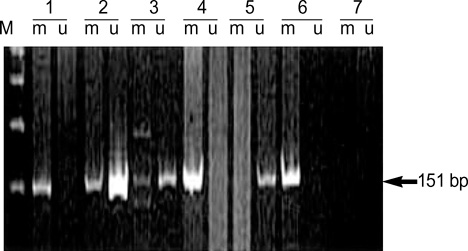
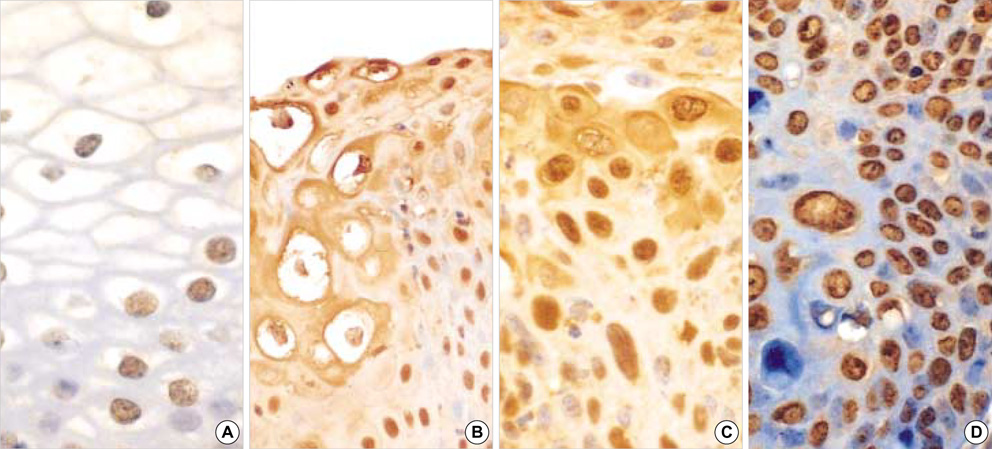
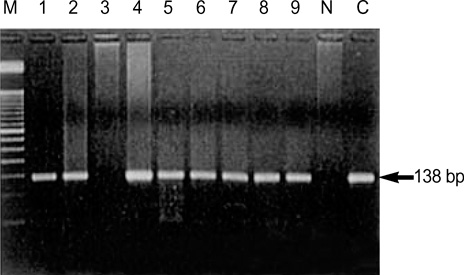
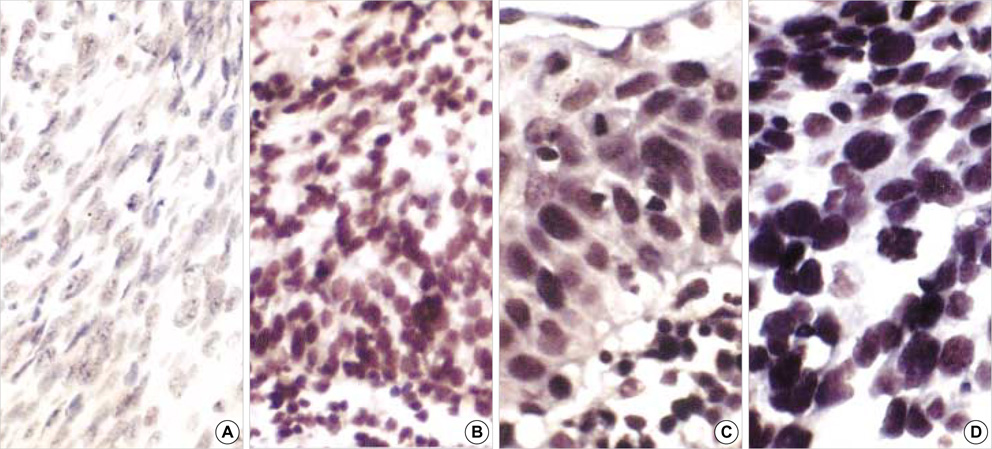
- Methylation of p16 is an important mechanism in cervical carcinogenesis. However, the relationship between cervical squamous cell carcinoma (SCC) and Epstein-Barr virus (EBV) remains controversial. Here, we explored whether EBV infection and/or p16 gene inactivation would play any role in cervical carcinogenesis. Eighty-two specimens included 41 invasive SCCs, 30 cervical intraepithelial neoplasm (CIN; CIN 1, 11 cases, CIN II, 3 cases, CIN III 16 cases) and 11 nonneoplastic cervices. EBV was detected by polymerase chain reaction (PCR) for EBNA-1 and in situ hybridization for EBER-1. The p16 methylation-status and the expression of p16 protein were studied by methylation-specific PCR and immunohistochemistry, respectively. The materials were divided into four groups: 1) nonneoplastic cervices, 2) CIN I, 3) CIN II-III and 4) invasive SCCs. p16 methylation and p16 immunoexpressions increased in CIN and invasive SCCs than nonneoplastic tissue. p16-methylation and p16-immunoreactivities were higher in the EBV-positive group (p=0.009, p<0.001) than in the EBV-negative group. EBV was detected more frequently in CIN and SCCs than nonneoplastic cervices. In conclusion, a correlation between p16 methylation, p16 immunoreactivity and the detection of EBV strongly suggested that the cooperation of EBV and p16 gene may play a synergic effect on cell cycle deregulation.
Keyword
MeSH Terms
-
Carcinoma, Squamous Cell/genetics/*pathology/virology
Comparative Study
Cyclin-Dependent Kinase Inhibitor p16/analysis/*genetics
*DNA Methylation
DNA, Viral/genetics/isolation & purification
Epstein-Barr Virus Infections/genetics/*pathology/virology
Epstein-Barr Virus Nuclear Antigens/genetics
Female
Herpesvirus 4, Human/genetics
Humans
Immunohistochemistry
In Situ Hybridization
Polymerase Chain Reaction
Precancerous Conditions/genetics/*pathology/virology
RNA, Viral/genetics
Research Support, Non-U.S. Gov't
Uterine Cervical Neoplasms/genetics/*pathology/virology
Figure
Reference
-
1. Nuovo GJ, Plaia TW, Belinsky SA, Baylin SB, Herman JG. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc Natl Acad Sci USA. 1999. 96:12754–12759.
Article2. Wong YF, Chung TK, Cheung TH, Nobori T, Yu AL, Yu J, Batova A, Lai KW, Chang AM. Methylation of p16INK4A in primary gynecologic malignancy. Cancer Lett. 1999. 136:231–235.3. Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995. 55:4525–4530.4. Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998. 153:1741–1748.
Article5. Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet. 2001. 10:687–692.
Article6. Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, Baylin SB, Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995. 1:686–692.
Article7. Keating JT, Ince T, Crum CP. Surrogate biomarkers of HPV infection in cervical neoplasia screening and diagnosis. Adv Anat Pathol. 2001. 8:83–92.
Article8. Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, Duensing S, Sheets EE, Munger K, Crum CP. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001. 25:884–891.9. Landers RJ, O'Leary JJ, Crowley M, Healy I, Annis P, Burke L, O'Brien D, Hogan J, Kealy WF, Lewis FA. Epstein-Barr virus in normal, pre-malignant and malignant lesions of the uterine cervix. J Clin Pathol. 1993. 46:931–935.
Article10. Taylor Y, Melvin WT, Sewell HF, Flannelly G, Walker F. Prevalence of Epstein-Barr virus in the cervix. J Clin Pathol. 1994. 47:92–93.
Article11. Sasagawa T, Shimakage M, Nakamura M, Sakaike J, Ishikawa H, Inoue M. Epstein-Barr virus (EBV) genes expression on cervical intraepithelial neoplasia and invasive cervical cancer: a comparative study with human papillomavirus (HPV) infection. Hum Pathol. 2000. 31:318–326.12. Noel J, Lespagnard L, Fayt I, Verhest A, Dargent J. Evidence of human papilloma virus infection but lack of Epstein-Barr virus in lymphoepithelioma-like carcinoma of uterine cervix: report of two cases and review of the literature. Hum Pathol. 2001. 32:135–138.
Article13. Shibosawa E, Tsutsumi K, Koizuka I, Hoshikawa M, Takakuwa T. Absence of nuclear p16 from Epstein-Barr virus-associated undifferentiated nasopharyngeal carcinomas. Laryngoscope. 2000. 110:93–97.
Article14. Schneider BG, Gulley ML, Eagan P, Bravo JC, Mera R, Geradts J. Loss of p16/CDKN2A tumor suppressor protein in gastric adenocarcinoma is associated with Epstein-Barr virus and anatomic location in the body of the stomach. Human Pathol. 2000. 31:45–50.
Article15. Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, Ro JY. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002. 160:787–794.
Article16. Cho NH, Kim YT, Kim JW. Alteration of cell cycle in cervical tumor associated with human Papillomavirus: Cyclin-dependent kinase inhibitors. Yonsei Med J. 2002. 43:722–728.
Article17. Kim IS, Kang JS, Choi AN, Kim YS. The prevalence of Epstein-Barr virus in uterine cervical cancer: Detection by PCR and in situ PCR methods. Korean J Obstet Gynecol. 2000. 43:184–191.18. Jeong HJ, Lee ES, Lin ZH, Park SH, Kim IS, Kang JS. Detection of Epstein-Barr virus in the inflammatory and neoplastic uterine cervical lesions. Korean J Cytopathol. 2001. 12:73–80.19. Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987. 19:11–15.20. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad USA. 1996. 93:9821–9826.
Article21. Geradts J, Kratzke RA, Niehans GA, Lincoln CE. Immunohistochemical detection of the cyclin-dependent kinase inhibitor 2/multiple tumor suppressor gene 1 (CDKN2/MTS1) product p16INK4A in archival human solid tumors: correlation with retinoblastoma protein expression. Cancer Res. 1995. 55:6006–6011.22. Coates PJ, d'Ardenne AJ, Khan G, Kangro HO, Slavin G. Simplified procedures for applying the polymerase chain reaction to routinely fixed paraffin wax sections. J Clin Pathol. 1991. 44:115–118.
Article23. Chang KL, Chen YY, Shibata D, Weiss LM. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol. 1992. 1:246–255.
Article24. Virmani AK, Muller C, Rathi A, Zoechbauer-Mueller S, Mathis M, Gazdar AF. Aberrant methylation during cervical carcinogenesis. Clin Cancer Res. 2001. 7:584–589.25. Wong YF, Chung TK, Cheung TH, Nobori T, Yim SF, Lai KW, Phil M, Yu AL, Diccianni MB, Li TZ, Chang AM. p16INK4 and p15INK4B alterations in primary gynecologic malignancy. Gynecol Oncol. 1997. 65:319–324.26. Salem C, Liang G, Tsai YC, Coulter J, Knowles MA, Feng AC, Groshen S, Nichols PW, Jones PA. Progressive increases in de novo methylation of CpG islands in bladder cancer. Cancer Res. 2000. 60:2473–2476.27. Yuen PW, Man M, Lam KY, Kwong YL. Clinicopathological significance of p16 gene expression in the surgical treatment of head and neck squamous cell carcinomas. J Clin Pathol. 2002. 55:58–60.28. Villuendas R, Sanchez-Beato M, Martinez JC, Saez AI, Martinez-Delgado B, Garcia JF, Mateo MS, Sanchez-Verde L, Benitez J, Martinez P, Piris MA. Loss of p16/INK4A protein expression in non-Hodgkin's lymphomas is a frequent finding associated with tumor progression. Am J Pathol. 1998. 153:887–897.
Article29. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, von Knebel Doeberitz M. Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001. 92:276–284.30. Nielsen GP, Stemmer-Rachamimov AO, Shaw J, Roy JE, Koh J, Louis DN. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest. 1999. 79:1137–1143.31. Shiozawa T, Nikaido T, Shimizu M, Zhai Y, Fujii S. Immunohistochemical analysis of the expression of cdk4 and p16INK4 in human endometrioid-type endometrial carcinoma. Cancer. 1997. 80:2250–2256.32. Timmermann S, Hinds PW, Munger K. Re-expression of endogenous p16ink4a in oral squamous cell carcinoma lines by 5-aza-2'-deoxycytidine treatment induces a senescence-like state. Oncogene. 1998. 17:3445–3453.33. Gump J, Koh J. An antibody to p16INK4A recognizes a modified form of galectin-3. Hybridoma. 2001. 20:167–174.34. Takeuchi H, Kobayashi R, Hasegawa M, Hirai K. Detection of latent Epstein-Barr virus (EBV) DNA in paraffin sections of nasopharyngeal carcinomas expressing no EBV-encoded small RNAs using in situ PCR. Arch Virol. 1997. 142:1743–1756.
Article35. Remus R, Kammer C, Heller H, Schmitz B, Schell G, Doerfler W. Insertion of foreign DNA into an established mammalian genome can alter the methylation of cellular DNA sequences. J Virol. 1999. 73:1010–1022.
Article36. Klein CB, Costa M. DNA methylation, heterochromatin and epigenetic carcinogens. Mutat Res. 1997. 386:163–180.
Article37. Toyooka S, Pass HI, Shivapurkar N, Fukuyama Y, Maruyama R, Toyooka KO, Gilcrease M, Farinas A, Minna JD, Gazdar AF. Aberrant methylation and simian virus 40 tag sequences in malignant mesothelioma. Cancer Res. 2001. 61:5727–5730.38. Reid GK, Besterman JM, MacLeod AR. Selective inhibition of DNA methyltransferase enzymes as a novel strategy for cancer treatment. Curr Opin Mol Ther. 2002. 4:130–137.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Epstein-Barr virus in the inflammatory and neoplastic uterine cervical lesions
- Alterations of p16INK4A Gene in Korean Squamous Cell Carcinoma and Basal Cell carcinoma of the Skin
- The Prevalence of Epstein-Barr Virus in Uterine Cervical Cancer: Detection by PCR and In Situ PCR Methods
- A case of lymphoepithelioma-like carcinoma of the uterine cervix
- Detection of Epstein-Barr Virus in Laryngeal Squamous Cell Carcinoma