J Gynecol Oncol.
2014 Apr;25(2):130-135. 10.3802/jgo.2014.25.2.130.
Metronomic oral paclitaxel shows anti-tumor effects in an orthotopic mouse model of ovarian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea. kimonc@hotmail.com
- 2Laboratory of Molecular Oncology, Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea.
- 3Human Resource Bank, Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea.
- 4Research & Development Center, Daehwa Pharm. Co., Hoengseong, Korea.
- KMID: 1708336
- DOI: http://doi.org/10.3802/jgo.2014.25.2.130
Abstract
OBJECTIVE
The purpose of this study was to compare the in vivo anti-tumor efficacy of a mucoadhesive, lipid-based, oral paclitaxel formulation (DHP107) with traditional, intraperitoneal (IP) paclitaxel using an orthotopic mouse model of chemotherapy-sensitive SKOV3ip1 ovarian cancer.
METHODS
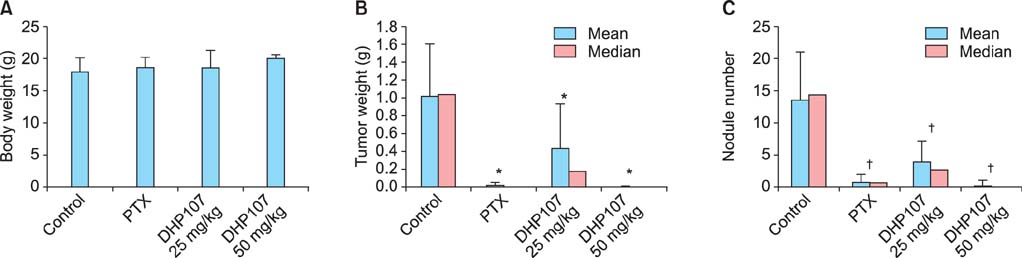
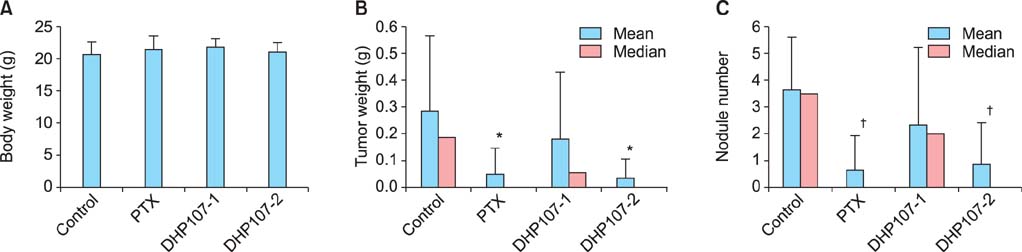
To determine the optimal therapeutic dose of oral paclitaxel, DHP107 was administered per os to female athymic nude mice at 0, 25, or 50 mg/kg twice per week. Control mice received 100 microL saline once per week. IP injections of paclitaxel at 5 mg/kg once per week were used for comparison. To evaluate the potential therapeutic effect of metronomic DHP107 chemotherapy, mice received DHP107 50 mg/kg once per week per os, which was compared with 25 mg/kg twice per week and with vehicle-treated controls.
RESULTS
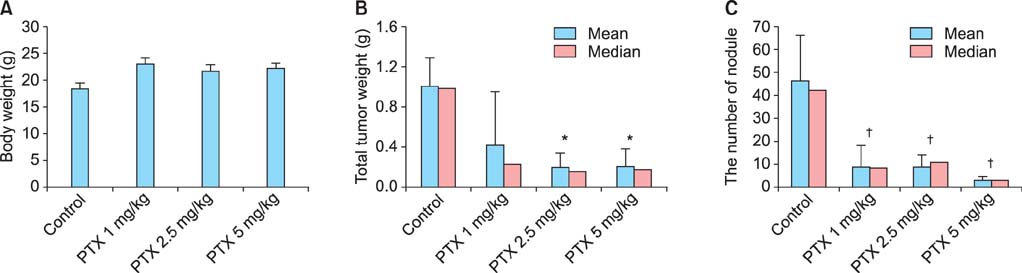
Low-dose DHP107 (25 mg/kg) twice per week was as effective as IP paclitaxel (5 mg/kg once a week) but high-dose DHP107 (50 mg/kg once per week) was less effective at inhibiting tumor growth in an orthotopic mouse model (88%, 82%, and 36% decrease in tumor weight, respectively). Mice that received 25 mg/kg DHP107 twice per week or 50 mg/kg DHP107 once per week per os had a significant decrease in tumor weight compared with vehicle-treated controls (p<0.01, both doses).
CONCLUSION
Metronomic oral chemotherapy with DHP107 showed anti-tumor efficacy in vivo similar to IP paclitaxel in an orthotopic mouse model.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim MG, Pak JH, Choi WH, Park JY, Nam JH, Kim JH. The relationship between cisplatin resistance and histone deacetylase isoform overexpression in epithelial ovarian cancer cell lines. J Gynecol Oncol. 2012; 23:182–189.2. Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009; 9:167–181.3. Kim MJ, Jung YW, Seong SJ, Yoon BS, Kim ML, Joo WD, et al. Intraoperative intraperitoneal chemotherapy with cisplatin in epithelial ovarian cancer. J Gynecol Oncol. 2012; 23:91–97.4. Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363:943–953.5. Kang HJ, Chang HM, Kim TW, Ryu MH, Sohn HJ, Yook JH, et al. A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer. Br J Cancer. 2008; 98:316–322.6. Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008; 26:5544–5552.7. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006; 355:2542–2550.8. Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990; 8:1263–1268.9. Liebmann J, Cook JA, Mitchell JB. Cremophor EL, solvent for paclitaxel, and toxicity. Lancet. 1993; 342:1428.10. Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008; 26:1642–1649.11. Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004; 4:423–436.12. Kamat AA, Kim TJ, Landen CN Jr, Lu C, Han LY, Lin YG, et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007; 67:281–288.13. Meerum Terwogt JM, Malingre MM, Beijnen JH, ten Bokkel Huinink WW, Rosing H, Koopman FJ, et al. Coadministration of oral cyclosporin A enables oral therapy with paclitaxel. Clin Cancer Res. 1999; 5:3379–3384.14. Shin BS, Kim HJ, Hong SH, Lee JB, Hwang SW, Lee MH, et al. Enhanced absorption and tissue distribution of paclitaxel following oral administration of DHP 107, a novel mucoadhesive lipid dosage form. Cancer Chemother Pharmacol. 2009; 64:87–94.15. Gao P, Rush BD, Pfund WP, Huang T, Bauer JM, Morozowich W, et al. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003; 92:2386–2398.16. Hong JW, Lee IH, Kwak YH, Park YT, Sung HC, Kwon IC, et al. Efficacy and tissue distribution of DHP107, an oral paclitaxel formulation. Mol Cancer Ther. 2007; 6(12 Pt 1):3239–3247.17. Landen CN, Kim TJ, Lin YG, Merritt WM, Kamat AA, Han LY, et al. Tumor-selective response to antibody-mediated targeting of alphavbeta3 integrin in ovarian cancer. Neoplasia. 2008; 10:1259–1267.18. Birner A. Safe administration of oral chemotherapy. Clin J Oncol Nurs. 2003; 7:158–162.19. Paine MF, Khalighi M, Fisher JM, Shen DD, Kunze KL, Marsh CL, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997; 283:1552–1562.20. Liggins RT, Hunter WL, Burt HM. Solid-state characterization of paclitaxel. J Pharm Sci. 1997; 86:1458–1463.21. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001; 37:1590–1598.22. Malingré MM, Beijnen JH, Rosing H, Koopman FJ, Jewell RC, Paul EM, et al. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer. 2001; 84:42–47.23. Britten CD, Baker SD, Denis LJ, Johnson T, Drengler R, Siu LL, et al. Oral paclitaxel and concurrent cyclosporin A: targeting clinically relevant systemic exposure to paclitaxel. Clin Cancer Res. 2000; 6:3459–3468.24. Veltkamp SA, Rosing H, Huitema AD, Fetell MR, Nol A, Beijnen JH, et al. Novel paclitaxel formulations for oral application: a phase I pharmacokinetic study in patients with solid tumours. Cancer Chemother Pharmacol. 2007; 60:635–642.25. Kerbel RS. Improving conventional or low dose metronomic chemotherapy with targeted antiangiogenic drugs. Cancer Res Treat. 2007; 39:150–159.26. Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000; 60:1878–1886.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An orthotopic nude mouse model of tongue carcinoma
- Efficacy of Paclitaxel-Loaded Bioadhesive Drug Deliver System Based on Glyceryl Monooleate Nanoparticle in an Orthotopic Murine Bladder Cancer Model
- The effects of selenium on tumor growth in epithelial ovarian carcinoma
- A Case of Paclitaxel Induced Scleroderma in a Patient with Ovarian Cancer
- Weekly versus 3-weekly paclitaxel in combination with carboplatin in advanced ovarian cancer: which is the optimal adjuvant chemotherapy regimen?