J Rheum Dis.
2011 Jun;18(2):110-113.
A Case of Paclitaxel Induced Scleroderma in a Patient with Ovarian Cancer
- Affiliations
-
- 1Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea. kimha@hallym.ac.kr
- 2Department of Dermatology, Hallym University Sacred Heart Hospital, Anyang, Korea.
Abstract
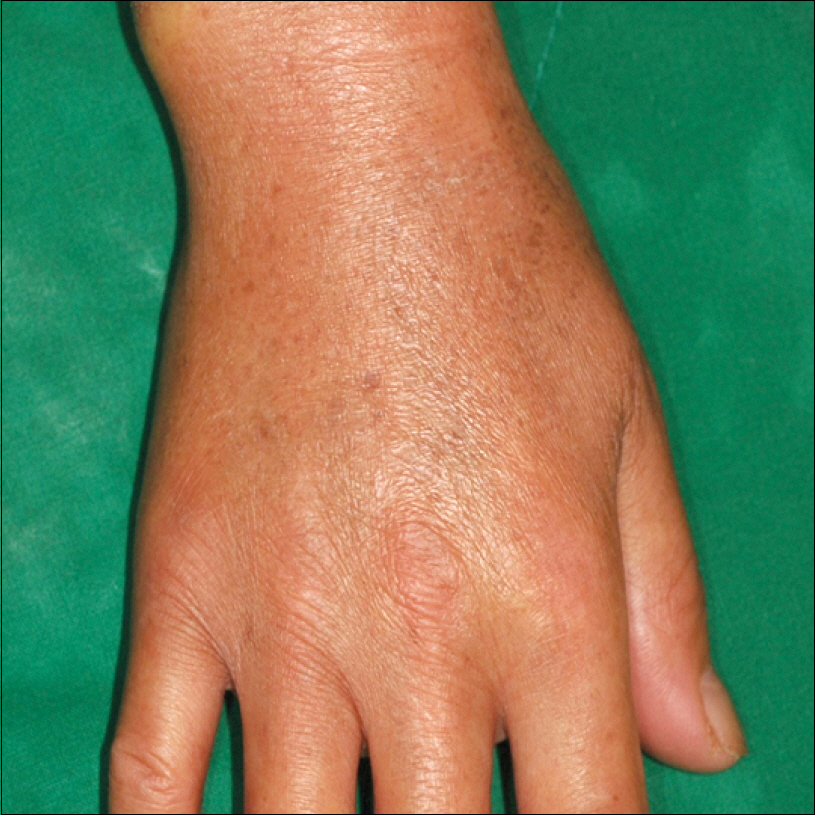
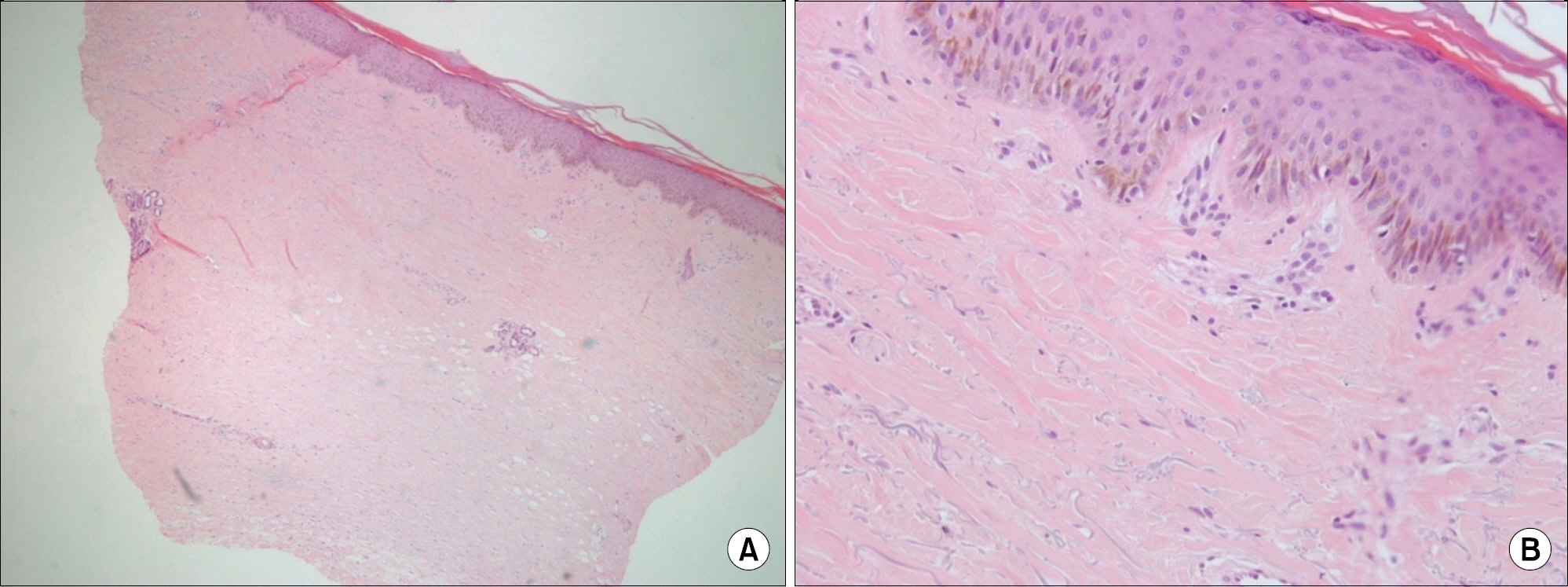
- Paclitaxel takes effects as an anti-neoplastic agent by interfering with microtubules and then blocking cell growth. It has been used to treat patients with lung, ovarian, breast, head and neck cancer, and advanced forms of Kaposi's sarcoma. Along with its reputation as an effective chemotherapeutic drug, paclitaxel has numerous adverse effects. Among them, cutaneous adverse effects of paclitaxel include pruritis, bullous fixed eruption, onycholysis, and transient erythrodysesthesia. Only several cases of scleroderma-like lesions have been reported throughout the world, and in Korea, only one case of paclitaxel induced scleroderma has been reported in 2006. We report a case of paclitaxel induced scleroderma in an 83-year old woman with ovarian cancer. After administration of paclitaxel and cisplatin, the patient presented with edema in both brachial areas, Raynaud's phenomenon, and sclerotic skin lesions in both extremities.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Pazdur R, Kudelka AP, Kavanagh JJ, Cohen PR, Raber MN. The taxoids: paclitaxel (Taxol) and docetaxel (Taxotere). Cancer Treat Rev. 1993; 19:351–86.
Article2. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol. 1993; 20(4 Suppl 3):1–15.3. Link CJ Jr, Sarosy GA, Kohn EC, Christian MC, Davis P, Adamo DO, et al. Cutaneous manifestations of Taxol therapy. Invest New Drugs. 1995; 13:261–3.
Article4. Itoh M, Yanaba K, Kobayashi T, Nakagawa H. Taxane-induced scleroderma. Br J Dermatol. 2007; 156:63–7.
Article5. Kono T, Ishii M, Negoro N, Taniguchi S. Scleroder-ma-like reaction induced by uracil-tegafur (UFT), a sec-ond-generation anticancer agent. J Am Acad Dermatol. 2000; 42:519–20.
Article6. Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992; 19:294–6.7. De Angelis R, Bugatti L, Cerioni A, Del Medico P, Filosa G. Diffuse scleroderma occurring after the use of paclitaxel for ovarian cancer. Clin Rheumatol. 2003; 22:49–52.
Article8. Yoon JH, Choi K, Seo HI, Lee YS, Oh SJ. A case of paclitaxel induced scleroderma. Korean J Med. 2006; 70:S373–6.9. Sato S. Abnormalities of adhesion molecules and che-mokines in scleroderma. Curr Opin Rheumatol. 1999; 11:503–7.
Article10. Bogdan C, Ding A. Taxol, a microtubule-stabilizing antineoplastic agent, induces expression of tumor necrosis factor alpha and interleukin-1 in macrophages. J Leukoc Biol. 1992; 52:119–21.11. Kawakami T, Tsutsumi Y, Soma Y. Limited cutaneous systemic sclerosis induced by paclitaxel in a patient with breast cancer. Arch Dermatol. 2009; 145:97–8.
Article12. Bielefeld P, Meyer P, Caillot D, Dalac S, Camus P, Tavernier C, et al. Systemic scleroderma and cancers: 21 cases and review of the literature. Rev Med Interne. 1996; 17:810–3.13. Sakkas LI, Moore DF, Akritidis NC. Cancer in families with systemic sclerosis. Am J Med Sci. 1995; 310:223–5.14. Young R, Towbin B, Isern R. Scleroderma and ovarian carcinoma. Br J Rheumatol. 1990; 29:314.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of advanced ovarian cancer which was treated with topotecan after taxol-cisplatin treatment failed
- A case of neoadjuvant chemotherapy with taxol / carboplatin in advanced epithelial ovarian cancer
- Weekly versus 3-weekly paclitaxel in combination with carboplatin in advanced ovarian cancer: which is the optimal adjuvant chemotherapy regimen?
- Effects of Osteopontin on Normal and Malignant Ovarian Epithelial Cell
- A case of bleomycin-induced scleroderma