Lab Anim Res.
2014 Jun;30(2):45-53. 10.5625/lar.2014.30.2.45.
Proteomic analysis of domestic pig pancreas during development using two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- Affiliations
-
- 1Laboratory of Developmental Biology and Genetics, College of Veterinary Medicine, BK21Plus Program for Creative Veterinary Science Research, BIO-MAX institute, Seoul National University, Seoul, Korea. snumouse@snu.ac.kr
- 2Department of Biomedical Laboratory Science, College of Biomedical Science, Soonchunhyang University, Asan, Korea.
- KMID: 1707443
- DOI: http://doi.org/10.5625/lar.2014.30.2.45
Abstract
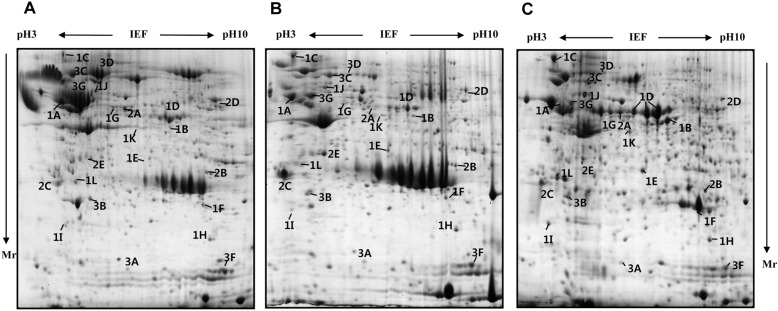
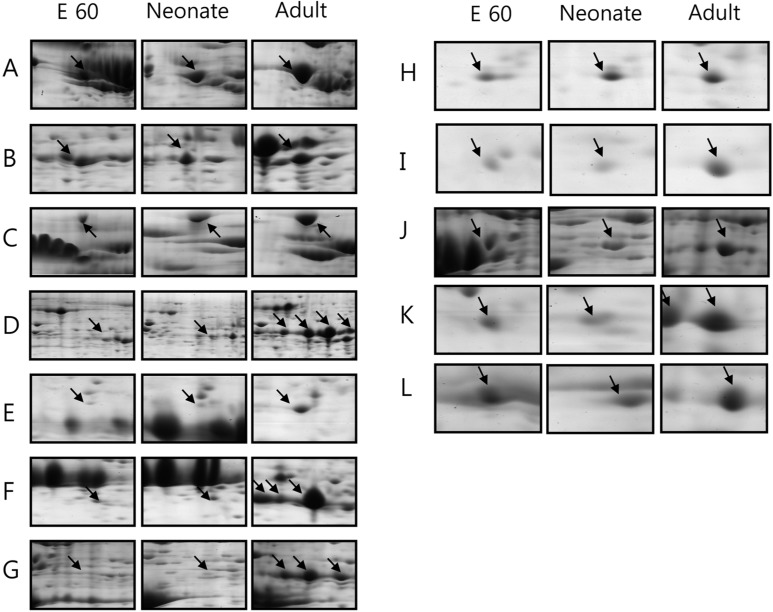
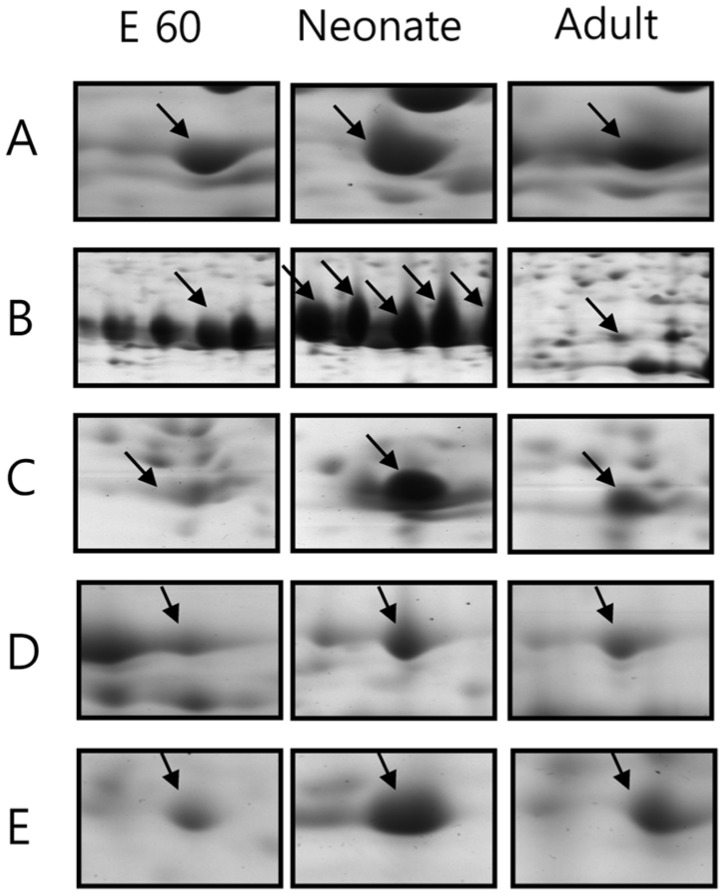
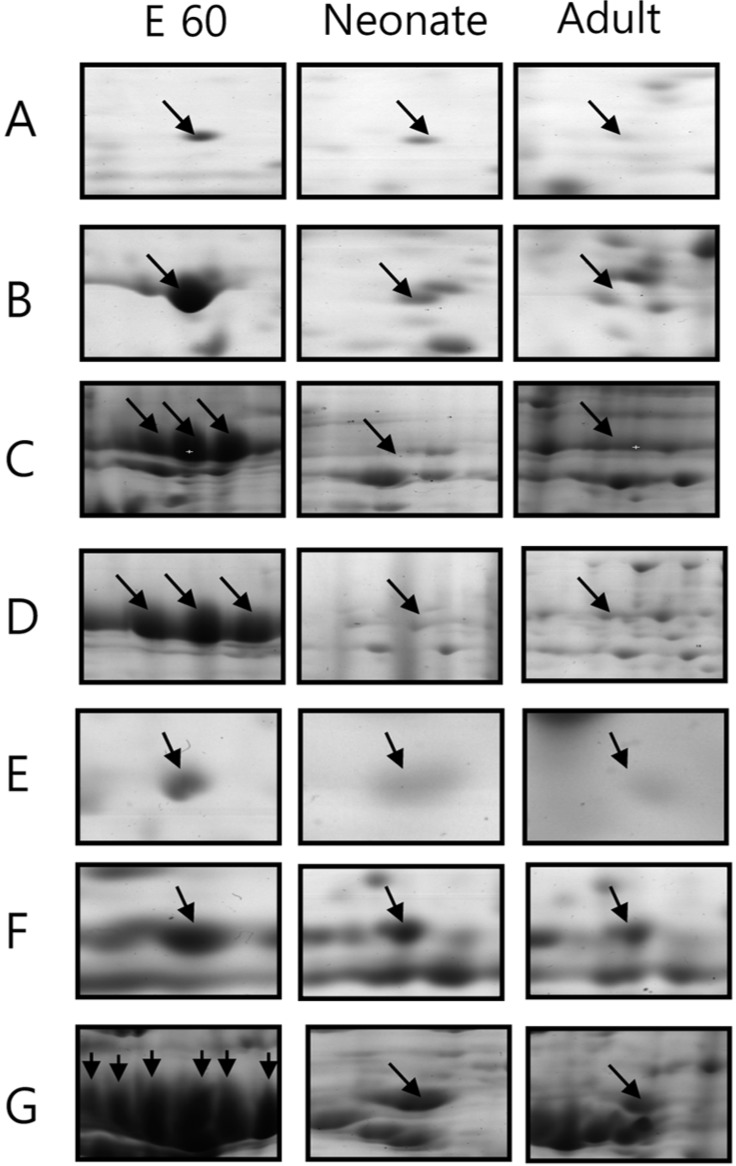
- Pig pancreas may be a therapeutic resource for human diabetic patients. However, this potential is hindered by a lack of knowledge of the molecular events of pig pancreas development. In this study, the embryonic day 60, neonate and 6-month protein profiles of pig pancreas were ascertained at using two-dimensional gel electrophoresis and matrix assisted laser desorption/ionization-time of flight mass spectrometry. Twenty four proteins were differentially expressed during pig pancreas development. Among them, 12 spots increased and 7 spots decreased according to development. The expression of 5 protein were highest at birth. Expression of digestive enzymes including trypsin, pancreatic triacylglycerol lipase and pancreatic alpha-amylase was elevated in adults, whereas chymotrypsins were highly expressed in neonates. Proteins that were abundantly expressed during gestation were alpha-1-antitrypsin, alpha-fetoprotein and transferrins. Taken together, we found out that several proteins were significantly up- or down- regulated from pig pancreas based on developmental stage. This study will provide basis for understanding development of pig pancreas.
Keyword
MeSH Terms
Figure
Reference
-
1. Rao MS, Reddy JK. Pancreatic stem cells: differentiation options. Stem Cell Rev. 2005; 1(3):265–271. PMID: 17142864.
Article2. Portela-Gomes GM, Hacker GW, Weitgasser R. Neuroendocrine cell markers for pancreatic islets and tumors. Appl Immunohistochem Mol Morphol. 2004; 12(3):183–192. PMID: 15551729.
Article3. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27(5):1047–1053. PMID: 15111519.
Article4. Herber S, Grus FH, Sabuncuo P, Augustin AJ. Two-dimensional analysis of tear protein patterns of diabetic patients. Electrophoresis. 2001; 22(9):1838–1844. PMID: 11425240.
Article5. Sabek OM, Marshall DR, Penmetsa R, Scarborough O, Gaber AO. Examination of gene expression profile of functional human pancreatic islets after 2-week culture. Transplant Proc. 2006; 38(10):3678–3679. PMID: 17175365.
Article6. Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007; 120(5):1046–1054. PMID: 17149698.
Article7. Højlund K, Wrzesinski K, Larsen PM, Fey SJ, Roepstorff P, Handberg A, Dela F, Vinten J, McCormack JG, Reynet C, Beck-Nielsen H. Proteome analysis reveals phosphorylation of ATP synthase beta-subunit in human skeletal muscle and proteins with potential roles in type 2 diabetes. J Biol Chem. 2003; 278(12):10436–10442. PMID: 12531894.8. Qiu L, List EO, Kopchick JJ. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics. 2005; 4(9):1311–1318. PMID: 15961380.
Article9. Silva D, Petrovsky N. Identification of key beta cell gene signaling pathways involved in type 1 diabetes. Ann N Y Acad Sci. 2004; 1037:203–207. PMID: 15699518.10. Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006; 13(6):488–499. PMID: 17059572.
Article11. Levy MF. Animal organs for human transplantation: how close are we? Proc (Bayl Univ Med Cent). 2000; 13(1):3–6. PMID: 16389317.
Article12. Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, Doyle H, Zeevi A, Warty V, Michaels M, Kusne S, Rudert WA, Trucco M. Baboon-to-human liver transplantation. Lancet. 1993; 341(8837):65–71. PMID: 8093402.
Article13. Levy MF, Crippin J, Sutton S, Netto G, McCormack J, Curiel T, Goldstein RM, Newman JT, Gonwa TA, Banchereau J, Diamond LE, Byrne G, Logan J, Klintmalm GB. Liver allotransplantation after extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers: clinical results and lack of pig-to-human transmission of the porcine endogenous retrovirus. Transplantation. 2000; 69(2):272–280. PMID: 10670638.14. Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, Chapman LE, Lockey C, Onions D, Otto E. The XEN 111 Study Group. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science. 1999; 285(5431):1236–1241. PMID: 10455044.
Article15. Steiner S, Anderson NL. Pharmaceutical proteomics. Ann N Y Acad Sci. 2000; 919:48–51. PMID: 11083096.
Article16. Görg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000; 21(6):1037–1053. PMID: 10786879.
Article17. Gobom J, Nordhoff E, Mirgorodskaya E, Ekman R, Roepstorff P. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J Mass Spectrom. 1999; 34(2):105–116. PMID: 10093212.
Article18. Erdjument-Bromage H, Lui M, Lacomis L, Grewal A, Annan RS, McNulty DE, Carr SA, Tempst P. Examination of micro-tip reversed-phase liquid chromatographic extraction of peptide pools for mass spectrometric analysis. J Chromatogr A. 1998; 826(2):167–181. PMID: 9871337.
Article19. Lainé J, Pelletier G, Grondin G, Peng M, LeBel D. Development of GP-2 and five zymogens in the fetal and young pig: biochemical and immunocytochemical evidence of an atypical zymogen granule composition in the fetus. J Histochem Cytochem. 1996; 44(5):481–499. PMID: 8627005.
Article20. Leblond FA, Morisset J, LeBel D. Alterations of pancreatic growth and of GP-2 content in the reserpinized rat model of cystic fibrosis. Pediatr Res. 1989; 25(5):478–481. PMID: 2717264.
Article21. Hayashi K, Pan Y, Shu H, Ohshima T, Kansy JW, White CL 3rd, Tamminga CA, Sobel A, Curmi PA, Mikoshiba K, Bibb JA. Phosphorylation of the tubulin-binding protein, stathmin, by Cdk5 and MAP kinases in the brain. J Neurochem. 2006; 99(1):237–250. PMID: 16925597.
Article22. Malorni L, Cacace G, Cuccurullo M, Pocsfalvi G, Chambery A, Farina A, Di Maro A, Parente A, Malorni A. Proteomic analysis of MCF-7 breast cancer cell line exposed to mitogenic concentration of 17beta-estradiol. Proteomics. 2006; 6(22):5973–5982. PMID: 17051647.23. Sewell DA, Yuan CX, Robertson E. Proteomic signatures in laryngeal squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec. 2007; 69(2):77–84. PMID: 17127822.
Article24. Zhang HZ, Wang Y, Gao P, Lin F, Liu L, Yu B, Ren JH, Zhao H, Wang R. Silencing stathmin gene expression by survivin promoter-driven siRNA vector to reverse malignant phenotype of tumor cells. Cancer Biol Ther. 2006; 5(11):1457–1461. PMID: 17012855.
Article25. Alli E, Yang JM, Hait WN. Silencing of stathmin induces tumor-suppressor function in breast cancer cell lines harboring mutant p53. Oncogene. 2007; 26(7):1003–1012. PMID: 16909102.
Article26. Ueda M, Hayase Y, Mashiba S. Establishment and evaluation of 2 monoclonal antibodies against oxidized apolipoprotein A-I (apoA-I) and its application to determine blood oxidized apoA-I levels. Clin Chim Acta. 2007; 378(1-2):105–111. PMID: 17174291.
Article27. Kim KD, Lim HY, Lee HG, Yoon DY, Choe YK, Choi I, Paik SG, Kim YS, Yang Y, Lim JS. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem Biophys Res Commun. 2005; 338(2):1126–1136. PMID: 16259956.
Article28. Zannis VI, Cole FS, Jackson CL, Kurnit DM, Karathanasis SK. Distribution of apolipoprotein A-I, C-II, C-III, and E mRNA in fetal human tissues. Time-dependent induction of apolipoprotein E mRNA by cultures of human monocyte-macrophages. Biochemistry. 1985; 24(16):4450–4455. PMID: 3931677.
Article29. Abelev GI. Alpha-fetoprotein as a marker of embryo-specific differentiations in normal and tumor tissues. Transplant Rev. 1974; 20(0):3–37. PMID: 4135841.
Article30. Cohen BL, Orn A, Gronvik KO, Gidlund M, Wigzell H, Murgita RA. Suppression by alpha-fetoprotein of murine natural killer cell activity stimulated in vitro and in vivo by interferon and interleukin 2. Scand J Immunol. 1986; 23(2):211–223. PMID: 2419966.
Article31. Murgita RA, Goidl EA, Kontiainen S, Beverley PC, Wigzell H. Adult murine T cells activated in vitro by alpha-fetoprotein and naturally occurring T cells in newborn mice: identity in function and cell surface differentiation antigens. Proc Natl Acad Sci U S A. 1978; 75(6):2897–2901. PMID: 78493.
Article32. Alpert E, Dienstag JL, Sepersky S, Littman B, Rocklin R. Immunosuppressive characteristics of human AFP: effect on tests of cell mediated immunity and induction of human suppressor cells. Immunol Commun. 1978; 7(2):163–185. PMID: 77249.33. Peck AB, Murgita RA, Wigzell H. Cellular and genetic restrictions in the immunoregulatory activity of alpha-fetoprotein.III. Role of the MLC-stimulating cell population in alpha-fetoprotein-induced suppression of T cell-mediated cytotoxicity. J Immunol. 1982; 128(3):1134–1140. PMID: 6173421.34. Murgita RA, Tomasi TB Jr. Suppression of the immune response by alpha-fetoprotein on the primary and secondary antibody response. J Exp Med. 1975; 141(2):269–286. PMID: 46267.
Article35. Yachnin S. Demonstration of the inhibitory effect of human alpha-fetoprotein on in vitro transformation of human lymphocytes. Proc Natl Acad Sci U S A. 1976; 73(8):2857–2861. PMID: 60761.
Article36. Auer IO, Kress HG. Suppression of the primary cell-mediated immune response by human alpha1-fetoprotein in vitro. Cell Immunol. 1977; 30(1):173–179. PMID: 67903.37. Littman BH, Alpert E, Rocklin RE. The effect of purified alpha-fetoprotein on in vitro assays of cell-mediated immunity. Cell Immunol. 1977; 30(1):35–42. PMID: 67905.38. Peck AB, Murgita RA, Wigzell H. Cellular and genetic restrictions in the immunoregulatory activity of alpha-fetoprotein. I. Selective inhibition of anti-Ia-associated proliferative reactions. J Exp Med. 1978; 147(3):667–683. PMID: 75940.
Article39. Olinescu A, Laky M, Pofescu DE, Dumitrescu A, Ganea D. The effect of alpha-fetoprotein on the immune response. III. Diminution of the phagocytosis capacity of macrophages cultured in vitro in the presence of mouse amniotic fluid or alpha-fetoprotein. Arch Roum Pathol Exp Microbiol. 1977; 36(3-4):247–253. PMID: 81666.40. The Shanghai coordinating group. Studies on alpha-fetoprotein v. influence of alpha-fetoprotein on immunological function. In advances in medical oncology, research, and education. In : Fox M, editor. Biological basis for cancer diagnosis. New York: Pergamon Press;1979. p. 297–301.41. Lu CY, Changelian PS, Unanue ER. Alpha-fetoprotein inhibits macrophage expression of Ia antigens. J Immunol. 1984; 132(4):1722–1727. PMID: 6199409.42. Crainie M, Semeluk A, Lee KC, Wegmann T. Regulation of constitutive and lymphokine-induced Ia expression by murine alpha-fetoprotein. Cell Immunol. 1989; 118(1):41–52. PMID: 2463096.43. Morris Buus R, Boockfor FR. Transferrin expression by placental trophoblastic cells. Placenta. 2004; 25(1):45–52. PMID: 15013638.
Article44. Briggs DA, Sharp DJ, Miller D, Gosden RG. Transferrin in the developing ovarian follicle: evidence for de-novo expression by granulosa cells. Mol Hum Reprod. 1999; 5(12):1107–1114. PMID: 10587364.
Article45. Lampreave F, Pineiro A. Concentrations of major plasma proteins in serum and whole-tissue extracts of porcine fetuses during development. J Reprod Fertil. 1992; 95(2):441–449. PMID: 1381439.
Article46. Nahreini P, Hovland AR, Kumar B, Andreatta C, Edwards-Prasad J, Prasad KN. Effects of altered cyclophilin A expression on growth and differentiation of human and mouse neuronal cells. Cell Mol Neurobiol. 2001; 21(1):65–79. PMID: 11440199.47. Jang G, Bhuiyan MM, Jeon HY, Ko KH, Park HJ, Kim MK, Kim JJ, Kang SK, Lee BC, Hwang WS. An approach for producing transgenic cloned cows by nuclear transfer of cells transfected with human alpha 1-antitrypsin gene. Theriogenology. 2006; 65(9):1800–1812. PMID: 16303172.
Article48. She QB, Mukherjee JJ, Crilly KS, Kiss Z. alpha(1)-antitrypsin can increase insulin-induced mitogenesis in various fibroblast and epithelial cell lines. FEBS Lett. 2000; 473(1):33–36. PMID: 10802054.49. Berdusco ET, Yang K, Challis JR, Hammond GL. Tissue distribution of alpha 1-proteinase inhibitor messenger ribonucleic acid and its regulation by glucocorticoids in fetal and neonatal sheep. Biol Reprod. 1993; 49(4):816–821. PMID: 8218647.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proteomic analysis of pancreas in miniature pigs according to developmental stages using two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- Identification of a Marker Protein for Cardiac Ischemia and Reperfusion Injury by Two-Dimensional Gel Electrophoresis and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry
- MALDI-TOF-MS Fingerprinting Provides Evidence of Urosepsis caused by Aerococcus urinae
- Risk of inaccurate species identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and of false carbapenem resistance by automated susceptibility analysis of Enterobacter spp.
- Application of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Imaging Mass Spectrometry (MALDI-TOF IMS) for Premalignant Gastrointestinal Lesions