Lab Anim Res.
2014 Mar;30(1):1-7. 10.5625/lar.2014.30.1.1.
Proteomic analysis of pancreas in miniature pigs according to developmental stages using two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Biomedical Sciences, Soonchunhyang University, Asan, Korea.
- 2Laboratory of Developmental Biology and Genetics, College of Veterinary Medicine, BK21 Plus Program for Creative Science Research, BIO-MAX institute, Seoul National University, Seoul, Korea. snumouse@snu.ac.kr
- 3Department of Pathology and Laboratory of Immune Regulation, College of Medicine, Seoul National University, Seoul, Korea.
- 4Transitional Research Institute, College of Medicine, Seoul National University, Seoul, Korea.
- 5Biomedical Center for Animal Resource and Development, BIO-MAX Institute, Seoul National University, Seoul, Korea.
- 6Department of Biochemistry, Hanyang University, Ansan, Korea.
- KMID: 2312113
- DOI: http://doi.org/10.5625/lar.2014.30.1.1
Abstract
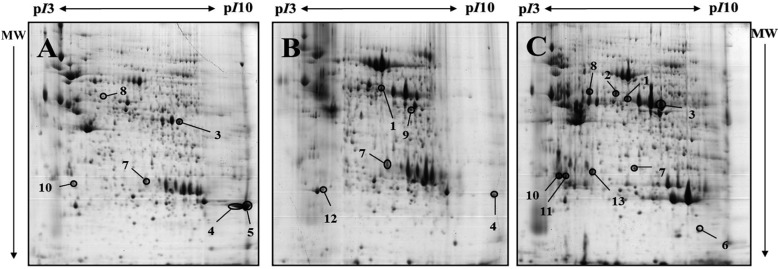
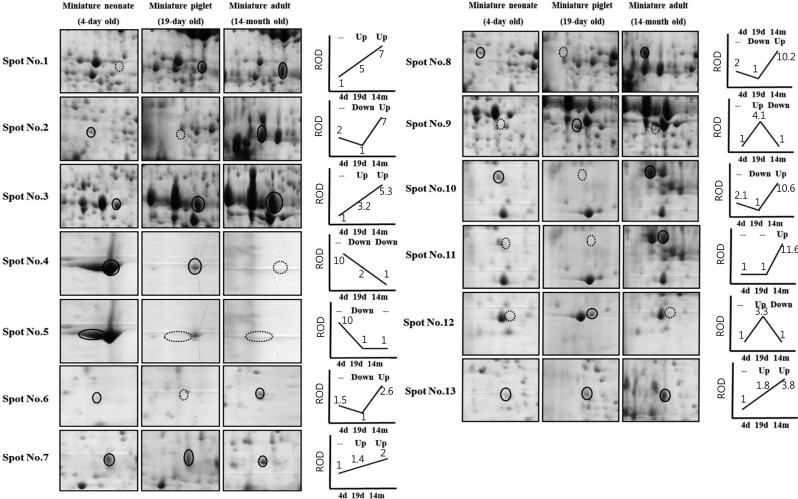
- Organ transplantation is limited by the shortage of human organs. Many studies have sought to overcome this hurdle by using animal organs. Porcine organs, especially from miniature pigs, have been used for organ xenotransplantation rather than nonhuman primates. While the molecular profiling for transplantation is well known in humans and rodents, the situation for pigs is almost completely unknown. The present study examined protein regulation of the developing stages of the pancreatic proteome (4 day-old miniature neonate, 19 day-old miniature piglet, and 14 month-old miniature adult pigs) using two-dimensional gel electrophoresis and matrix assisted laser desorption/ionization-time of flight mass spectrometry. Thirteen different expressed spots were observed and nine were identified. The data presented within this study provides critical direction relating to the development of pancreas of miniature pigs, which will assist future proteome analysis of the pancreas, and advance our understanding of the hurdles facing xenotransplantation.
Keyword
MeSH Terms
Figure
Reference
-
1. Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002; 53:133–147. PMID: 11818467.
Article2. Platt JL. Physiologic barriers to xenotransplantation. Transplant Proc. 2000; 32(7):1547–1548. PMID: 11119828.
Article3. Ryu JM, Kim DH, Lee MY, Lee SH, Park JH, Yun SP, Jang MW, Kim SH, Rho GJ, Han HJ. Imaging evaluation of the liver using multi-detector row computed tomography in micropigs as potential living liver donors. J Vet Sci. 2009; 10(2):93–98. PMID: 19461203.
Article4. Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006; 13(6):488–499. PMID: 17059572.
Article5. Tucker A, Belcher C, Moloo B, Bell J, Mazzulli T, Humar A, Hughes A, McArdle P, Talbot A. The production of transgenic pigs for potential use in clinical xenotransplantation: baseline clinical pathology and organ size studies. Xenotransplantation. 2002; 9(3):203–208. PMID: 11983018.
Article6. Ando A, Ota M, Sada M, Katsuyama Y, Goto R, Shigenari A, Kawata H, Anzai T, Iwanaga T, Miyoshi Y, Fujimura N, Inoko H. Rapid assignment of the swine major histocompatibility complex (SLA) class I and II genotypes in Clawn miniature swine using PCR-SSP and PCR-RFLP methods. Xenotransplantation. 2005; 12(2):121–126. PMID: 15693842.
Article7. Park CG, Kim JS, Shin JS, Kim YH, Kim SJ. Current Status and Future Perspectives of xenotransplantation. J Korean Soc Transplant. 2009; 23(3):203–213.8. Vodicka P, Smetana K Jr, Dvoránková B, Emerick T, Xu YZ, Ourednik J, Ourednik V, Motlík J. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci. 2005; 1049:161–171. PMID: 15965115.9. Choi JS, Cho YK, Yoon SH, Kwon SO, Koo DB, Yu K. Proteomic analysis of porcine pancreas development. BMB Rep. 2009; 42(10):661–666. PMID: 19874711.
Article10. Han HJ, Kang SS, Park SH. Tissue specific expression of lipid metabolism related molecules in digestive organs of miniature pigs. Lab Anim Res. 2010; 26(3):273–278.
Article11. Lee MS, Song KD, Yang HJ, Solis CD, Kim SH, Lee WK. Development of a type II diabetic mellitus animal model using Micropig®. Lab Anim Res. 2012; 28(3):205–208. PMID: 23091521.
Article12. Cozzi E, Bosio E. Islet xenotransplantation: current status of preclinical studies in the pig-to-nonhuman primate model. Curr Opin Organ Transplant. 2008; 13(2):155–158. PMID: 18685296.
Article13. Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep. 2011; 11(5):402–412. PMID: 21805400.
Article14. Rood PP, Buhler LH, Bottino R, Trucco M, Cooper DK. Pig-to-nonhuman primate islet xenotransplantation: a review of current problems. Cell Transplant. 2006; 15(2):89–104. PMID: 16719044.
Article15. Steiner S, Anderson NL. Pharmaceutical proteomics. Ann N Y Acad Sci. 2000; 919:48–51. PMID: 11083096.
Article16. Görg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000; 21(6):1037–1053. PMID: 10786879.
Article17. Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996; 68(5):850–858. PMID: 8779443.18. Lowe ME. The triglyceride lipases of the pancreas. J Lipid Res. 2002; 43(12):2007–2016. PMID: 12454260.
Article19. Mahan JT, Heda GD, Rao RH, Mansbach CM 2nd. The intestine expresses pancreatic triacylglycerol lipase: regulation by dietary lipid. Am J Physiol Gastrointest Liver Physiol. 2001; 280(6):G1187–G1196. PMID: 11352812.
Article20. Hagenbüchle O, Bovey R, Young RA. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980; 21(1):179–187. PMID: 6157477.21. Doyon Y, Home W, Daull P, LeBel D. Effect of C-domain N-glycosylation and deletion on rat pancreatic alpha-amylase secretion and activity. Biochem J. 2002; 362(Pt 1):259–264. PMID: 11829764.22. Miyata S, Akazawa T. Biosynthesis of rice seed alpha-amylase: proteolytic processing and glycosylation of precursor polypeptides by microsomes. J Cell Biol. 1983; 96(3):802–806. PMID: 6187753.
Article23. Miyata S, Akazawa T. alpha-Amylase biosynthesis: evidence for temporal sequence of NH2-terminal peptide cleavage and protein glycosylation. Proc Natl Acad Sci USA. 1982; 79(21):6566–6568. PMID: 6183665.
Article24. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004; 4(7):499–511. PMID: 15229469.
Article25. Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006; 27(8):352–357. PMID: 16807108.
Article26. Subramaniam S, Stansberg C, Cunningham C. The interleukin 1 receptor family. Dev Comp Immunol. 2004; 28(5):415–428. PMID: 15062641.
Article27. Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005; 17(1):1–14. PMID: 15585605.28. Elzinga BM, Twomey C, Powell JC, Harte F, McCarthy JV. Interleukin-1 receptor type 1 is a substrate for gamma-secretase-dependent regulated intramembrane proteolysis. J Biol Chem. 2009; 284(3):1394–1409. PMID: 18996842.29. Abcouwer SF, Norman J, Fink G, Carter G, Lustig RJ, Souba WW. Tissue-specific regulation of glutamine synthetase gene expression in acute pancreatitis is confirmed by using interleukin-1 receptor knockout mice. Surgery. 1996; 120(2):255–263. PMID: 8751591.
Article30. Chentouf M, Dubois G, Jahannaut C, Castex F, Lajoix AD, Gross R, Peraldi-Roux S. Excessive food intake, obesity and inflammation process in Zucker fa/fa rat pancreatic islets. PLoS One. 2011; 6(8):e22954. PMID: 21826222.
Article31. Fink GW, Norman JG. Specific changes in the pancreatic expression of the interleukin 1 family of genes during experimental acute pancreatitis. Cytokine. 1997; 9(12):1023–1027. PMID: 9417814.32. Subramanian A, Miller DM. Structural analysis of alpha-enolase. Mapping the functional domains involved in down-regulation of the c-myc protooncogene. J Biol Chem. 2000; 275(8):5958–5965. PMID: 10681589.33. Wold F. Boyer PD, editor. The Enzymes. Ed. 5. Academic Press: New York, USA;1971. p. 499–508.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proteomic analysis of domestic pig pancreas during development using two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- Identification of a Marker Protein for Cardiac Ischemia and Reperfusion Injury by Two-Dimensional Gel Electrophoresis and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry
- MALDI-TOF-MS Fingerprinting Provides Evidence of Urosepsis caused by Aerococcus urinae
- Risk of inaccurate species identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and of false carbapenem resistance by automated susceptibility analysis of Enterobacter spp.
- Application of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Imaging Mass Spectrometry (MALDI-TOF IMS) for Premalignant Gastrointestinal Lesions