Ann Clin Microbiol.
2014 Mar;17(1):1-8. 10.5145/ACM.2014.17.1.1.
European Strategies to Control Antibiotic Resistance and Use
- Affiliations
-
- 1Laboratory of Medical Microbiology, Vaccine and Infectious Diseases Institute, University of Antwerp and University Hospital Antwerp, Edegem, Belgium. Herman.Goossens@uza.be
- KMID: 1518097
- DOI: http://doi.org/10.5145/ACM.2014.17.1.1
Abstract
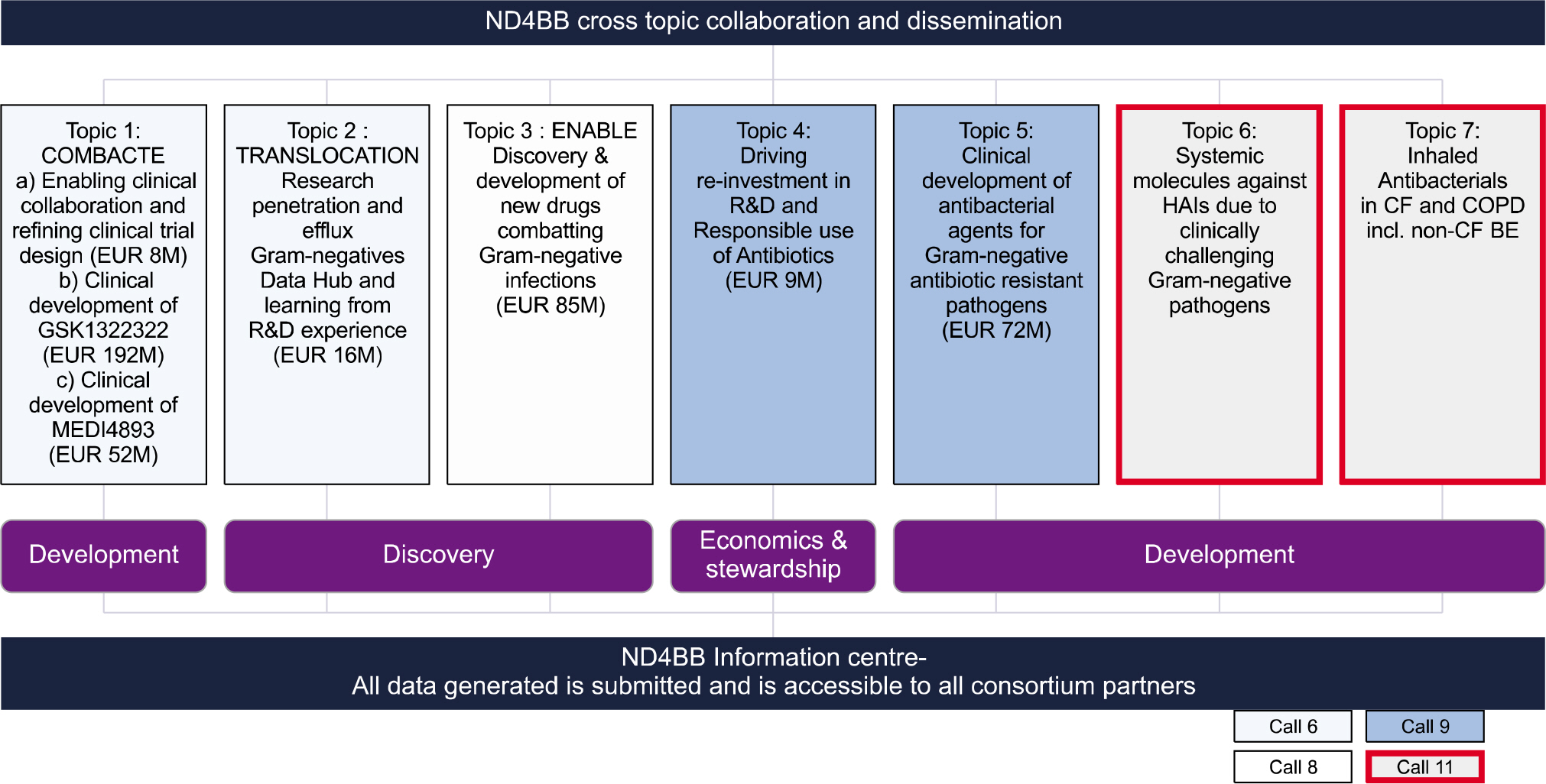
- Europe has taken many political actions since 1999 to better control antimicrobial resistance and use, including two European Council Recommendations and actions taken by numerous European Union (EU) presidencies. These presidencies triggered many public health and research actions in the EU. Europe developed several very successful surveillance programmes on antimicrobial resistance and antimicrobial use, both currently coordinated by the European Centre for Disease Prevention and Control (ECDC). These surveillance programmes were able to identify emerging problems of antibiotic resistance and targets for quality improvement of antimicrobial use; they also conducted impact assessments of campaigns to reduce antibiotic use and increase hand hygiene. The public antibiotic awareness campaigns were very successful in reducing antibiotic use and resistance in countries like Belgium and France. The successes of these campaigns inspired ECDC to launch an annual European Antibiotic Awareness Day on November 18, 2008. The hand hygiene campaigns resulted in a dramatic decrease of MRSA infections in many EU Member States. However, ESBL-producing Gram-negative bacteria and Carbapenem-resistant Enterobacteriaceae and non-fermenters are increasing in most EU countries. Finally, the EU is investing hundreds of millions of EUROs in a Public Private Partnership (PPP), called the Innovative Medicines Initiative (IMI). An important initiative of IMI is the launch of the Combating Antibiotic Resistance NewDrugs4BadBugs programme. The goal of this new research programme is to create an innovative and collaborative PPP-based approach that will positively impact all aspects of the antimicrobial resistance issue, from the discovery of novel products to Phase 1-3 clinical trials.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of Antibiotic Resistance Rate of Medically Important Microorganisms between Japan and Korea
Keigo Shibayama, Hyukmin Lee, Sunjoo Kim
Ann Clin Microbiol. 2015;18(4):111-118. doi: 10.5145/ACM.2015.18.4.111.
Reference
-
1.The Copenhagen Recommendations. Report from the Invitational EU Conference on the Microbial Threat, Copenhagen, Denmark, 9-10 September 1998. http://soapimg.icecube.snowfall.se/strama/Kopenham-nsmotet_1998.pdf/[Online. (last visited on 13 November, 2013).2.Council of the European Union. Council Recommendation of 9 June 2009 on Patient Safety. (2009/C 151/01).http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0001:0006:EN:PDF/. [Online] (last visited on 13 November, 2013).3.Monnet D., Kristinsson K. Turning the tide of antimicrobial resistance: Europe shows the way. Euro Surveill. 2008. 13(46):pii:19039.
Article4.Goossens H. Expert-proposed European strategies to monitor and control infection, antibiotic use, and resistance in health-care facilities. Lancet Infect Dis. 2011. 11:338–40.
Article5.National Institute of Public Health and the Environment, Bilthoven, The Netherlands. EARSS - European Antimicrobial Resistance Surveillance System. Annual Report 2001.http://ecdc.europa.eu/en/activities/surveillance/EARS-Net/Documents/2001_EARSS_Annual_Report.pdf/. [Online] (last visited on 13 November, 2013).6.ECDC. Surveillance Report - Antimicrobial resistance surveillance in Europe (2009). http://ecdc.europa.eu/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf/[Online. (last visited on 13 November, 2013).7.Bronzwaer SL., Buchholz U., Kool JL. International surveillance of antimicrobial resistance in Europe: now we also need to monitor antibiotic use. Euro Surveill. 2001. 6:1–2.
Article8.European Council. Council recommendation of 15 November 2001 on the prudent use of antimicrobial agents in human medicine. http://www.hpa.org.uk/cdr/archives/2004/cdr2904.pdf. [Online] (last isited on 17 March 2014).9.Vander Stichele RH., Elseviers MM., Ferech M., Blot S., Goossens H. ESAC Project Group. European surveillance of antimicrobial consumption (ESAC): data collection performance and methodolo-gical approach. Br J Clin Pharmacol. 2004. 58:419–28.
Article10.European Surveillance of Antimicrobial Consumption: the ESAC programme. Euro Surveill 2004. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2518. [Online] (last isited on 17 March 2014).11.Ferech M., Coenen S., Dvorakova K., Hendrickx E., Suetens C., Goossens H. ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): outpatient penicillin use in Europe. J Antimicrob Chemother. 2006. 58:408–12.
Article12.Muller A., Coenen S., Monnet DL., Goossens H. ESAC Project Grou. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe, 1998-2005. Euro Surveill. 2007. 12:E071011.1.
Article13.Adriaenssens N., Coenen S., Muller A., Vankerckhoven V., Goossens H. ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): outpatient systemic antimycotic and antifungal use in Europe. J Antimicrob Chemother. 2010. 65:769–74.
Article14.Ferech M., Coenen S., Malhotra-Kumar S., Dvorakova K., Hendrickx E., Suetens C, et al. ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): outpatient quinolone use in Europe. J Antimicrob Chemother. 2006. 58:423–7.
Article15.Coenen S., Ferech M., Dvorakova K., Hendrickx E., Suetens C., Goossens H. ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): outpatient cephalosporin use in Europe. J Antimicrob Chemother. 2006. 58:413–7.
Article16.Coenen S., Ferech M., Malhotra-Kumar S., Hendrickx E., Suetens C., Goossens H. ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): outpatient macrolide, lincosa-mide and streptogramin (MLS) use in Europe. J Antimicrob Chemother. 2006. 58:418–22.
Article17.Coenen S., Muller A., Adriaenssens N., Vankerckhoven V., Hendrickx E., Goossens H. ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): outpatient parenteral antibiotic treatment in Europe. J Antimicrob Chemother. 2009. 64:200–5.
Article18.Goossens H., Ferech M., Coenen S., Stephens P. European Surveillance of Antimicrobial Consumption Project Group. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin Infect Dis. 2007. 44:1091–5.
Article19.Kuster SP., Ruef C., Ledergerber B., Hintermann A., Deplazes C., Neuber L, et al. Quantitative antibiotic use in hospitals: comparison of measurements, literature review, and recommendations for a standard of reporting. Infection. 2008. 36:549–59.
Article20.Amadeo B., Zarb P., Muller A., Drapier N., Vankerckhoven V., Rogues AM, et al. ESAC III Hospital Care Subproject Group. European Surveillance of Antibiotic Consumption (ESAC) point prevalence survey 2008: paediatric antimicrobial prescribing in 32 hospitals of 21 European countries. J Antimicrob Chemother. 2010. 65:2247–52.
Article21.Zarb P., Amadeo B., Muller A., Drapier N., Vankerckhoven V., Davey P, et al. ESAC-3 Hospital Care Subproject Group. Identi-fication of targets for quality improvement in antimicrobial prescribing: the web-based ESAC Point Prevalence Survey 2009. J Antimicrob Chemother. 2011. 66:443–9.22.Henderson KL., Müller-Pebody B., Johnson AP., Goossens H., Sharland M. ARPEC Group. First set-up meeting for Antibiotic Resistance and Prescribing in European Children (ARPEC). http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19404. [Online] (last isited on 17 March 2014).23.Goossens H., Coenen S., Costers M., De Corte S., De Sutter A., Gordts B, et al. Achievements of the Belgian Antibiotic Policy Coordination Committee (BAPCOC). Euro Surveill 2008.http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19036. [Online] (last isited on 17 March 2014).24.Goossens H., Guillemot D., Ferech M., Schlemmer B., Costers M., van Breda M, et al. National campaigns to improve antibiotic use. Eur J Clin Pharmacol. 2006. 62:373–9.
Article25.Sabuncu E., David J., Bernède-Bauduin C., Pépin S., Leroy M., Boëlle PY, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 2009. 6:e1000084.
Article26.Huttner B., Harbarth S. "Antibiotics are not automatic anymore"--the French national campaign to cut antibiotic overuse. PLoS Med. 2009. 6:e1000080.
Article27.Huttner B., Goossens H., Verheij T., Harbarth S. CHAMP consortium. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010. 10:17–31.
Article28.Earnshaw S., Monnet DL., Duncan B., O'Toole J., Ekdahl K., Goossens H. European Antibiotic Awareness Day Technical Advisory Committee; European Antibiotic Awareness Day Collaborative Group. European Antibiotic Awareness Day, 2008-the first Euro-pe-wide public information campaign on prudent antibiotic use: methods and survey of activities in participating countries. Euro Surveill. 2009. 14:19280.
Article29.Abdul Ghafur K. An obituary--on the death of antibiotics! J Assoc Physicians India. 2010. 58:143–4.30.Hughes JM. Preserving the lifesaving power of antimicrobial agents. JAMA. 2011. 305:1027–8.
Article31.Norrby SR., Nord CE., Finch R. European Society of Clinical Microbiology and Infectious Diseases. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005. 5:115–9.
Article32.Spellberg B., Miller LG., Kuo MN., Bradley J., Scheld WM., Edwards JE Jr. Societal costs versus savings from wild-card patent extension legislation to spur critically needed antibiotic development. Infection. 2007. 35:167–74.
Article33.Kaplan W., Laing R. Priority Medicines for Europe and the World. WHO, Geneva, Switzerland.http://archives.who.int/prioritymeds/report/final18october.pdf/[Online. (last visited on 13 November, 2013).34.Transatlantic Taskforce on Antimicrobial Resistance. Recommendations for future collaboration between the U.S. and EU. 2011.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Myths and Misconceptions around Antibiotic Resistance: Time to Get Rid of Them
- Antibiotic Resistance of Helicobacter pylori: Mechanisms and Clinical Implications
- The Worldwide Antibiotic Campaigns
- Antibiotic Stewardship: A Key Strategy to Combat Antibiotic Resistance
- Antibiotic Resistance and Treatment Update of Community-Acquired Pneumonia