Yonsei Med J.
2013 Mar;54(2):416-424. 10.3349/ymj.2013.54.2.416.
Long-Term (Postnatal Day 70) Outcome and Safety of Intratracheal Transplantation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Neonatal Hyperoxic Lung Injury
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wonspark@skku.edu
- 2Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Korea Institute of Toxicology, Daejeon, Korea.
- 4Biomedical Research Institute, MEDIPOST Co., Ltd., Seoul, Korea.
- KMID: 1503905
- DOI: http://doi.org/10.3349/ymj.2013.54.2.416
Abstract
- PURPOSE
This study was performed to evaluate the long-term effects and safety of intratracheal (IT) transplantation of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) in neonatal hyperoxic lung injury at postnatal day (P)70 in a rat model.
MATERIALS AND METHODS
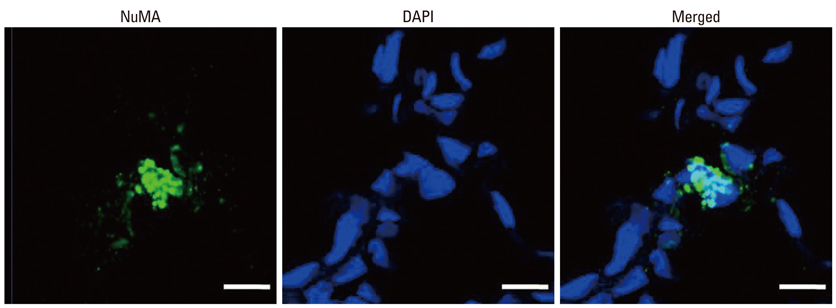
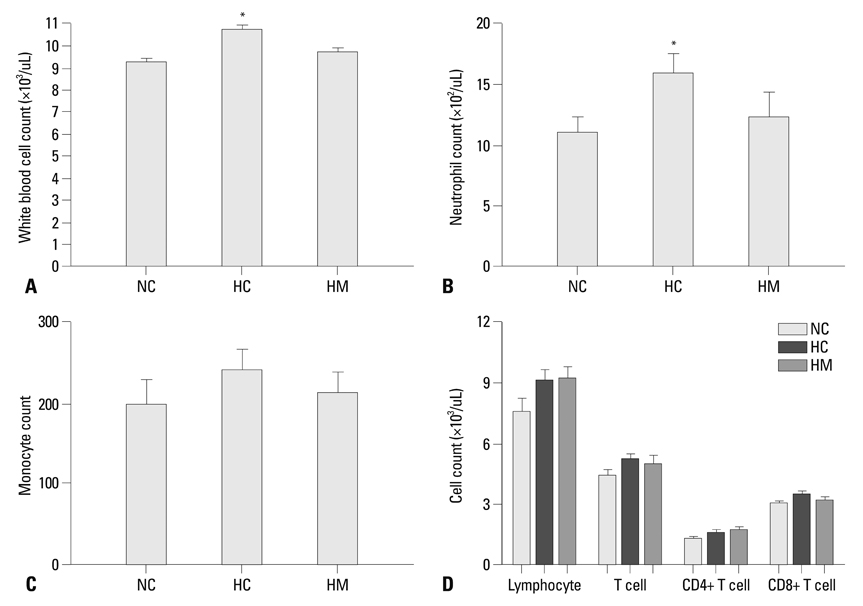
Newborn Sprague Dawley rat pups were subjected to 14 days of hyperoxia (90% oxygen) within 10 hours after birth and allowed to recover at room air until sacrificed at P70. In the transplantation groups, hUCB-MSCs (5x10(5)) were administered intratracheally at P5. At P70, various organs including the heart, lung, liver, and spleen were histologically examined, and the harvested lungs were assessed for morphometric analyses of alveolarization. ED-1, von Willebrand factor, and human-specific nuclear mitotic apparatus protein (NuMA) staining in the lungs and the hematologic profile of blood were evaluated.
RESULTS
Impaired alveolar and vascular growth, which evidenced by an increased mean linear intercept and decreased amount of von Willebrand factor, respectively, and the hyperoxia-induced inflammatory responses, as evidenced by inflammatory foci and ED-1 positive alveolar macrophages, were attenuated in the P70 rat lungs by IT transplantation of hUCB-MSCs. Although rare, donor cells with human specific NuMA staining were persistently present in the P70 rat lungs. There were no gross or microscopic abnormal findings in the heart, liver, or spleen, related to the MSCs transplantation.
CONCLUSION
The protective and beneficial effects of IT transplantation of hUCB-MSCs in neonatal hyperoxic lung injuries were sustained for a prolonged recovery period without any long-term adverse effects up to P70.
Keyword
MeSH Terms
-
Animals
*Cord Blood Stem Cell Transplantation
Ectodysplasins/metabolism
Humans
Hyperoxia/*pathology
Lung/metabolism/pathology
Lung Injury/pathology/*surgery
*Mesenchymal Stem Cell Transplantation
Models, Animal
Nuclear Matrix-Associated Proteins/metabolism
Rats
Trachea/*transplantation
von Willebrand Factor/metabolism
Ectodysplasins
Nuclear Matrix-Associated Proteins
von Willebrand Factor
Figure
Cited by 1 articles
-
Stem Cell Therapy for Neonatal Disorders: Prospects and Challenges
Yun Sil Chang, So Yoon Ahn, Sein Sung, Won Soon Park
Yonsei Med J. 2017;58(2):266-271. doi: 10.3349/ymj.2017.58.2.266.
Reference
-
1. Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol. 2006. 30:219–226.
Article2. Bland RD. Neonatal chronic lung disease in the post-surfactant era. Biol Neonate. 2005. 88:181–191.
Article3. Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics. 1987. 79:26–30.
Article4. Bregman J, Farrell EE. Neurodevelopmental outcome in infants with bronchopulmonary dysplasia. Clin Perinatol. 1992. 19:673–694.
Article5. Chang YS, Choi SJ, Sung DK, Kim SY, Oh W, Yang YS, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011. 20:1843–1854.
Article6. Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, et al. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009. 18:869–886.
Article7. Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005. 6:872–884.
Article8. Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007. 109:1743–1751.
Article9. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2--intrauterine and early postnatal lung growth. Thorax. 1982. 37:580–583.
Article10. Takada H, Kishimoto C, Hiraoka Y. Therapy with immunoglobulin suppresses myocarditis in a murine coxsackievirus B3 model. Antiviral and anti-inflammatory effects. Circulation. 1995. 92:1604–1611.
Article11. Smith S, Liggitt D, Jeromsky E, Tan X, Skerrett SJ, Wilson CB. Local role for tumor necrosis factor alpha in the pulmonary inflammatory response to Mycobacterium tuberculosis infection. Infect Immun. 2002. 70:2082–2089.
Article12. Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L143–L152.
Article13. Baraldi E, Filippone M, Trevisanuto D, Zanardo V, Zacchello F. Pulmonary function until two years of life in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1997. 155:149–155.
Article14. Warner BB, Stuart LA, Papes RA, Wispé JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998. 275(1 Pt 1):L110–L117.15. Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, et al. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L529–L535.
Article16. Lau M, Masood A, Yi M, Belcastro R, Li J, Tanswell AK. Long-term failure of alveologenesis after an early short-term exposure to a PDGF-receptor antagonist. Am J Physiol Lung Cell Mol Physiol. 2011. 300:L534–L547.
Article17. Choo-Wing R, Nedrelow JH, Homer RJ, Elias JA, Bhandari V. Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007. 293:L142–L150.
Article18. Lindsay L, Oliver SJ, Freeman SL, Josien R, Krauss A, Kaplan G. Modulation of hyperoxia-induced TNF-alpha expression in the newborn rat lung by thalidomide and dexamethasone. Inflammation. 2000. 24:347–356.19. Marcho Z, White JE, Higgins PJ, Tsan MF. Tumor necrosis factor enhances endothelial cell susceptibility to oxygen toxicity: role of glutathione. Am J Respir Cell Mol Biol. 1991. 5:556–562.
Article20. Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol. 2008. 34:174–190.
Article21. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003. 31:890–896.
Article22. Bartholomew A, Patil S, Mackay A, Nelson M, Buyaner D, Hardy W, et al. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum Gene Ther. 2001. 12:1527–1541.
Article23. Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001. 29:244–255.
Article24. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005. 105:1815–1822.
Article25. Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009. 11:377–391.
Article26. Tocci A, Forte L. Mesenchymal stem cell: use and perspectives. Hematol J. 2003. 4:92–96.
Article27. Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000. 342:1846–1854.
Article28. Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, et al. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004. 6:476–486.
Article29. Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003. 5:485–489.
Article30. Northway WH Jr, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990. 323:1793–1799.
Article31. Mourani PM, Sontag MK, Kerby GS, Fashaw L, Abman SH. Persistent impairment of lung function during infancy correlates with severity of bronchopulmonary dysplasia at diagnosis. Am J Respir Crit Care Med. 2010. 181:A3931.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- Intratracheal Administration of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in a Patient with Acute Respiratory Distress Syndrome
- Intratracheal Transplantation of Amnion-Derived Mesenchymal Stem Cells Ameliorates Hyperoxia-Induced Neonatal Hyperoxic Lung Injury via Aminoacyl-Peptide Hydrolase
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Study on the Culture and in vivo transplantation of Mesenchymal Stem Cells derived from Human Umbilical Cord Blood