J Bacteriol Virol.
2013 Dec;43(4):297-306. 10.4167/jbv.2013.43.4.297.
Cytoplasmic Translocation of p53 by Human Cytomegalovirus Infection
- Affiliations
-
- 1Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea. hesss@snu.ac.kr
- 2BK21 Plus Biomedical Science Project, Seoul National University College of Medicine, Seoul, Korea.
- 3Institute of Endemic Diseases, Seoul National University Medical Research Center, Seoul, Korea.
- KMID: 1501998
- DOI: http://doi.org/10.4167/jbv.2013.43.4.297
Abstract
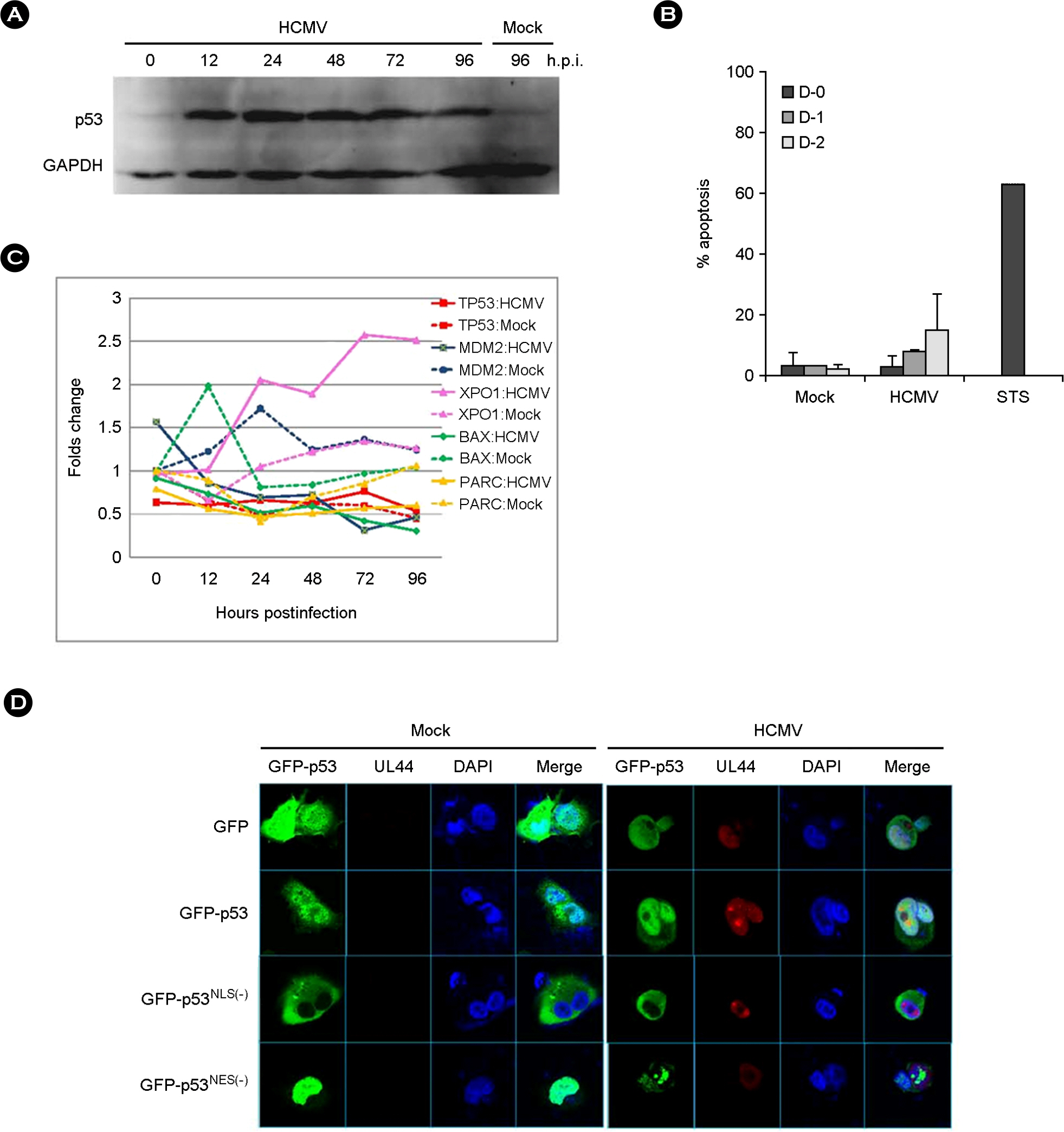
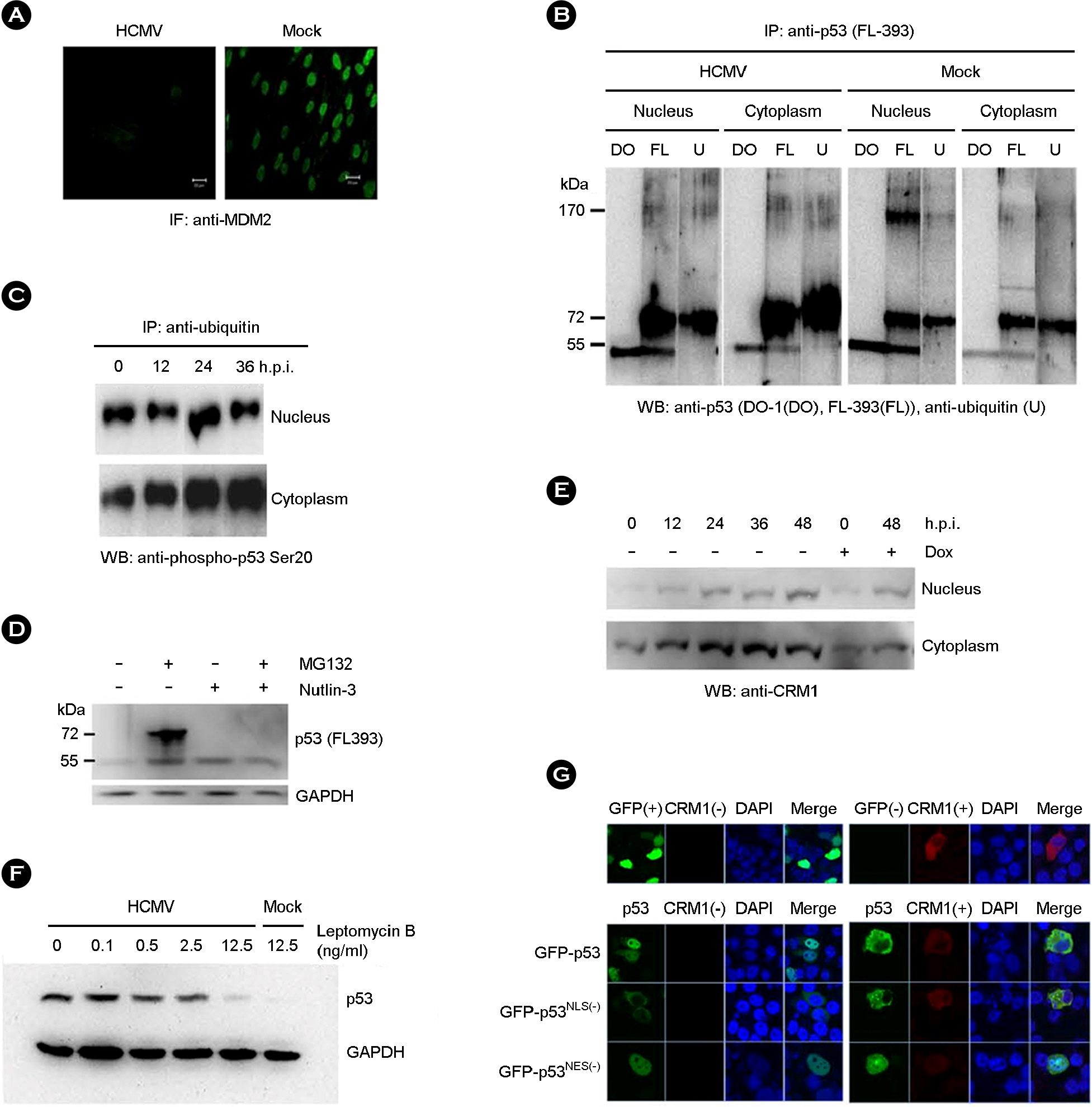
- p53 is a well-known multi-functional transcription regulator and is critical in the induction of apoptosis in response to various stresses. Human cytomegalovirus (HCMV) infection induced the accumulation of p53, which was partly relocalized in cytoplasm, but no apparent cell death in human fibroblasts. p53 in HCMV-infected cells was mainly mono-ubiquitinated, which might be resulted from the decreased expression of MDM2 in the course of HCMV infection. Ubiquitinated p53 was also phosphorylated at serine 20. CRM1 increased in the cytoplasm of HCMV-infected cells. It was found that p53 and its mutant in nuclear export sequences were localized in the cytoplasm of cells when co-expressed with CRM1. Collectively, our data suggest that HCMV infection modifies p53 into a stable and exportable form and accumulates it in the cytoplasm, but does not result in apoptotic death of cells.
Keyword
MeSH Terms
Figure
Reference
-
1). Moll UM, Schramm LM. p53–an acrobat in tumorigenesis. Crit Rev Oral Biol Med. 1998; 9:23–37.
Article2). Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009; 458:1127–30.
Article3). Brooks CL, Li M, Gu W. Monoubiquitination: the signal for p53 nuclear export? Cell Cycle. 2004; 3:436–8.
Article4). Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol. 2007; 9:428–35.
Article5). Halaby MJ, Yang DQ. p53 translational control: a new facet of p53 regulation and its implication for tumorigenesis and cancer therapeutics. Gene. 2007; 395:1–7.
Article6). Linzer DI, Levine AJ. Characterization of a 54 K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979; 17:43–52.7). Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979; 278:261–3.8). Sarnow P, Ho YS, Williams J, Levine AJ. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982; 28:387–94.
Article9). Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990; 63:1129–36.
Article10). Muganda P, Mendoza O, Hernandez J, Qian Q. Human cytomegalovirus elevates levels of the cellular protein p53 in infected fibroblasts. J Virol. 1994; 68:8028–34.
Article11). Castillo JP, Yurochko AD, Kowalik TF. Role of human cytomegalovirus immediate-early proteins in cell growth control. J Virol. 2000; 74:8028–37.
Article12). Speir E, Modali R, Huang ES, Leon MB, Shawl F, Finkel T, et al. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994; 265:391–4.
Article13). Hwang ES, Zhang Z, Cai H, Huang DY, Huong SM, Cha CY, et al. Human cytomegalovirus IE1-72 protein interacts with p53 and inhibits p53-dependent transactivation by a mechanism different from that of IE2-86 protein. J Virol. 2009; 83:12388–98.
Article14). Kwon Y, Kim MN, Young Choi E, Heon Kim J, Hwang ES, Cha CY. Inhibition of p53 transcriptional activity by human cytomegalovirus UL44. Microbiol Immunol. 2012; 56:324–31.
Article15). Casavant NC, Luo MH, Rosenke K, Winegardner T, Zurawska A, Fortunato EA. Potential role for p53 in the permissive life cycle of human cytomegalovirus. J Virol. 2006; 80:8390–401.
Article16). Utama B, Shen YH, Mitchell BM, Makagiansar IT, Gan Y, Muthuswamy R, et al. Mechanisms for human cytomegalovirus-induced cytoplasmic p53 sequestration in endothelial cells. J Cell Sci. 2006; 119:2457–67.
Article17). Shao H, Yi XM, Wells A. Epidermal growth factor protects fibroblasts from apoptosis via PI3 kinase and Rac signaling pathways. Wound Repair Regen. 2008; 16:551–8.
Article18). Kudo N, Wolff B, Sekimoto T, Schreiner EP, Yoneda Y, Yanagida M, et al. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998; 242:540–7.
Article19). Jault FM, Jault JM, Ruchti F, Fortunato EA, Clark C, Corbeil J, et al. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J Virol. 1995; 69:6697–704.
Article20). Kovacs A, Weber ML, Burns LJ, Jacob HS, Vercellotti GM. Cytoplasmic sequestration of p53 in cytomegalovirus-infected human endothelial cells. Am J Pathol. 1996; 149:1531–9.21). Wang J, Belcher JD, Marker PH, Wilcken DE, Vercellotti GM, Wang XL. Cytomegalovirus inhibits p53 nuclear localization signal function. J Mol Med. 2001; 78:642–7.
Article22). Chen Z, Knutson E, Wang S, Martinez LA, Albrecht T. Stabilization of p53 in human cytomegalovirus-initiated cells is associated with sequestration of HDM2 and decreased p53 ubiquitination. J Biol Chem. 2007; 282:29284–95.
Article23). Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, et al. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J Virol. 2004; 78:10960–6.
Article24). Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997; 91:325–34.
Article25). Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci U S A. 1999; 96:13777–82.
Article26). Momand J, Wu HH, Dasgupta G. MDM2–master regulator of the p53 tumor suppressor protein. Gene. 2000; 242:15–29.27). Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000; 275:8945–51.
Article28). Zhang Z, Evers DL, McCarville JF, Dantonel JC, Huong SM, Huang ES. Evidence that the human cytomegalovirus IE2-86 protein binds mdm2 and facilitates mdm2 degradation. J Virol. 2006; 80:3833–43.
Article29). Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003; 302:1972–5.
Article30). Bond JA, Webley K, Wyllie FS, Jones CJ, Craig A, Hupp T, et al. p53-Dependent growth arrest and altered p53-immunoreactivity following metabolic labelling with 32P ortho-phosphate in human fibroblasts. Oncogene. 1999; 18:3788–92.
Article31). Craig AL, Burch L, Vojtesek B, Mikutowska J, Thompson A, Hupp TR. Novel phosphorylation sites of human tumour suppressor protein p53 at Ser20 and Thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers. Biochem J. 1999; 342:133–41.
Article32). Stephen CW, Helminen P, Lane DP. Characterisation of epitopes on human p53 using phage-displayed peptide libraries: insights into antibody-peptide interactions. J Mol Biol. 1995; 248:58–78.
Article33). Chan WM, Mak MC, Fung TK, Lau A, Siu WY, Poon RY. Ubiquitination of p53 at multiple sites in the DNA-binding domain. Mol Cancer Res. 2006; 4:15–25.
Article34). Kastan MB, Zambetti GP. Parc-ing p53 in the cytoplasm. Cell. 2003; 112:1–2.
Article35). Lohrum MA, Woods DB, Ludwig RL, Bálint E, Vousden KH. C-terminal ubiquitination of p53 contributes to nuclear export. Mol Cell Biol. 2001; 21:8521–32.
Article36). Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999; 18:1660–72.
Article37). Zhang Y, Xiong Y. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science. 2001; 292:1910–5.
Article38). Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997; 90:1051–60.
Article39). Okorokov AL, Rubbi CP, Metcalfe S, Milner J. The interaction of p53 with the nuclear matrix is mediated by F-actin and modulated by DNA damage. Oncogene. 2002; 21:356–67.
Article40). Bakhanashvili M, Novitsky E, Lilling G, Rahav G. P53 in cytoplasm may enhance the accuracy of DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase. Oncogene. 2004; 23:6890–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Translocation of p53 Protein in Melanocytes and Malignant Melanoma Cells After UVB Irradiation
- Two cases of congenital cytomegalovirus infection
- A study of neonatal cholestasis and cytomegalovirus infection
- Two Cases of Prenatal Diagnosis of Congenital Cytomegalovirus Infection
- Cytomegalovirus infection in patients with HIV infection