J Bacteriol Virol.
2008 Sep;38(3):161-166. 10.4167/jbv.2008.38.3.161.
Effect of Culture Conditions on the Protein Fibril Expression of Candida albicans
- Affiliations
-
- 1Department of Microbiology, Yonsei University Wonju College of Medicine and Institute of Basic Medicine, Yonsei University, Wonju, Korea. shinws@kwandong.ac.kr
- 2Department of Anatomy, Yonsei University Wonju College of Medicine and Institute of Basic Medicine, Yonsei University, Wonju, Korea.
- 3Department of Microbiology, Kwandong University College of Medicine, Gangneung, Korea.
- KMID: 1483974
- DOI: http://doi.org/10.4167/jbv.2008.38.3.161
Abstract
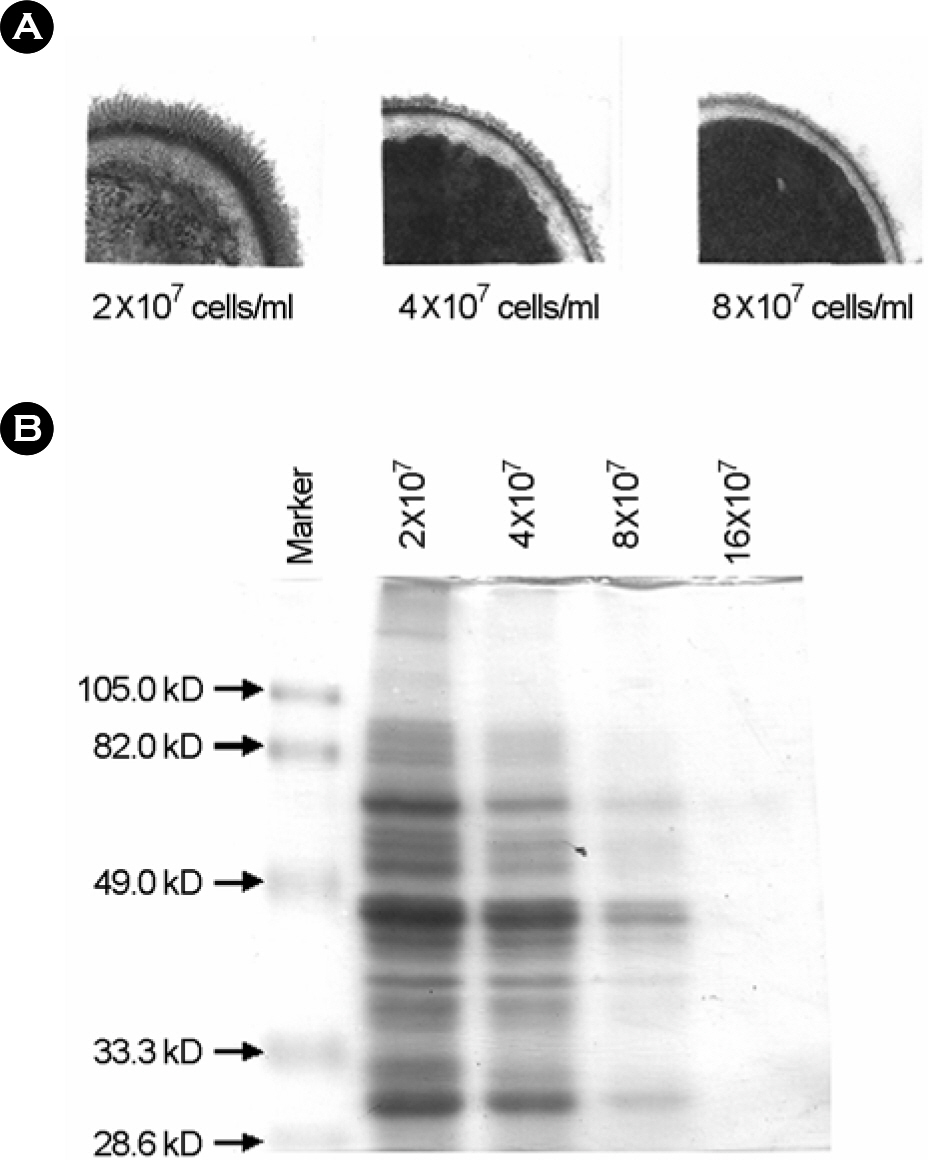
- Candida albicans is an important human pathogen that causes systemic infections, predominantly among population with weakened immune system. Cell wall structures of C. albicans are important to adhere to host tissue and evade to host immune system. Among cell wall structure, the outermost fibrillar layer of C. albicans is of interest since it may play important roles in antigenicity, phagocytosis, and adherence. The expression of virulent factors could be affected by the growth conditions. The dynamic nature of the cell surface alters the physical properties of the fungal interface with host cells and thereby influences adhesion to the host and recognition by components of the host immune system. In this study, we investigated the effects of culture conditions on cell surface fibril expression of C. albicans by a transmitting electron microscopy and SDS-PAGE. The protein fibril of C. albicans was expressed in the presence of whole serum, however, the fibril expression was decreased in 25% serum and serum containing 1% glucose. Also, germ tube can be induced by serum, RMPI medium, N-acetyl glucosamine, and 39 degrees C culture condition, hence, the fibrillar structure of C. albicans was detected only in serum-induced germ tube. The expression of fibril layer and the major fibril proteins of 66, 47, 30 kDa were reduced as increasing cell concentration of intial inoculum from 2x10(7) cells/ml to 8x10(7) cells/ml. The fibrillar layer of C. albicans was expressed in serum early within 10 min, and the thickness of fibril layer was increased according to the increase of culture time. When the fibrillar proteins were analysed by SDS-PAGE, major protein of 30 kDa was maintained continuously during over night culture although expression of the other proteins were various. These results suggest that expression of serum induced protein fibril is influenced by culture conditions and is not related to hyphal transition of C. albicans.
Keyword
MeSH Terms
Figure
Reference
-
1). Agabian N., Odds FC., Poulain D., Soll DR., White TC. Pathogenesis of invasive candidiasis. J Med Vet Mycol. 32:S229–237. 1994.
Article2). Antley PP., Hazen KC. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect Immun. 56:2884–2890. 1988.3). Beveridge TJ., Popkin TJ., Cole RM. Electron microscopy. pp. p. 42–71. In. Methods for general and molecular bacteriology. Gerhardt P, Murray RGE, Wood WA, Krieg NR, editors. (Ed),. American society for microbiology;Washington DC: 1994.
Article4). Bradford MM. A rapid, sensitive method for the quantitation of micrograme quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. 1976.5). Brawner DL., Cutler JE. Ultrastructural and biochemical studies of two dynamically expressed cell surgace determinants on Candida albicans. Infect Immun. 51:327–336. 1986.6). Calderone RA., Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 9:327–335. 2001.7). Calderone RA., Linehan L., Wadsworth E., Sandberg AL. Identification of C3d receptors on Candida albicans. Infect Immun. 56:252–258. 1988.8). Cutler JE. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 45:187–218. 1991.9). Enoch DA., Ludlam HA., Brown NM. Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol. 55:809–818. 2006.
Article10). Glee PM., Sundstrom P., Hazen KC. Expression of surface hydrophobic proteins by Candida albicans in vivo. Infect Immun. 63:1373–1379. 1995.11). Hazen KC., Hazen BW. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Infect Immun. 60:1499–1508. 1992.12). Hilmioglu S., Ilkit M., Badak Z. Comparison of 12 liquid media for germ tube production of Candida albicans and C. tropicalis. Mycoses. 50:282–285. 2007.13). Hubbard MJ., Sullivan PA., Shepherd MG. Morphological studies of N-acetylglucosamine induced germ tube formation by Candida albicans. Can J Microbiol. 31:696–701. 1985.14). Kanbe T., Han Y., Redgrave B., Riesselman MH., Cutler JE. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph nodes tissue. Infect Immun. 61:2578–2584. 1993.15). Kennedy MJ., Sandin RL. Influence of growth conditions on Candida albicans adhesion, hydrophobicity and cell wall ultrastructure. J Med Vet Mycol. 26:79–92. 1988.16). Kim D., Shin WS., Lee KH., Kim K., Park JY., Koh CM. Rapid differentiation of Candida albicans from other Candida species using its unique germ tube formation at 39 degrees C. Yeast. 19:957–962. 2002.17). Koh CM., Lee KH., Shin WS., Kim DH. Serum induces protein fibrils expression in Candida albicans. J Korean Soc Microbiol. 34:277–283. 1999.18). Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. 1970.
Article19). Lehrer N., Segal E., Lis H., Gov Y. Effect of Candida abicans cell wall components on the adhesion of the fungus to human and murine vaginal mucosa. Mycopathologia. 102:115–121. 1988.20). Monod M., Borg-von Zepelin M. Secreted proteinases and other virulence mechanisms of Candida albicans. Chem Immunol. 81:114–128. 2002.21). Odds FC. Candida species and virulence. ASM News. 60:313–318. 1994.22). Odds FC. Disseminated candidosis (Candida septicemia). pp. p. 206–230. Candida and candidiosis. 2nd ed.Odds FC, editor. (Ed),. WB Saunders;London: 1988.23). Odds FC. Morphogenesis in Candida albicans. Crit Rev Microbiol. 12:45–93. 1985.24). Osumi M. The ultrastructure of yeast: cell wall structure and formation. Micron. 29:207–233. 1998.
Article25). Pagano L., Caira M., Candoni A., Offidani M., Fianchi L., Martino B., Pastore D., Picardi M., Bonini A., Chierichini A., Fanci R., Caramatti C., Invernizzi R., Mattei D., Mitra ME., Melillo L., Aversa F., Van Lint MT., Falcucci P., Valentini CG., Girmenia C., Nosari A. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 91:1068–1075. 2006.26). Pendrak ML., Krutzsch HC., Roberts DD. Structural requirements for hemoglobin to induce fibronectin receptor expression in Candida albicans. Biochemistry. 39:16110–16118. 2000.27). Ruiz-Herrera J., Elorza MV., Valentin E., Sentandreu R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenecity. FEMS Yeast Res. 6:14–29. 2006.28). Sakata N., Yamazaki K., Koqure T. Identification of a 21 kDa laminin-binding component of Candida albicans. Zentralbl Bakteriol. 289:217–25. 1999.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genes Expression of Human Monocytes in Response to Candida albicans by Microarray and Its Clinical Application
- Ultrastructure of Fibrillar Layer of Candida albicans in Serum Culture
- In vitro Evaluation of the Antifungal Activity of Propolis Extract on Cryptococcus neoformans and Candida albicans
- In Vitro Antifungal Activity of Equol against Candida albicans
- Identification of Candida Species Using CHROMagar Candida in Superficial Cutaneous Candidiasis