J Bacteriol Virol.
2008 Dec;38(4):179-188. 10.4167/jbv.2008.38.4.179.
Epidemiological Prevalence of Avian Pathogenic Escherichia coli Differentiated by Multiplex PCR from Commercial Chickens and Hatchery in Korea
- Affiliations
-
- 1Department of Infectious Diseases and Avian Diseases, College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, Jeonju, Korea. hkjang@chonbuk.ac.kr
- 2Department of Physiology, College of Medicine and Bio-Food and Drug Research Center, Konkuk University, Chungju, Korea.
- KMID: 1483958
- DOI: http://doi.org/10.4167/jbv.2008.38.4.179
Abstract
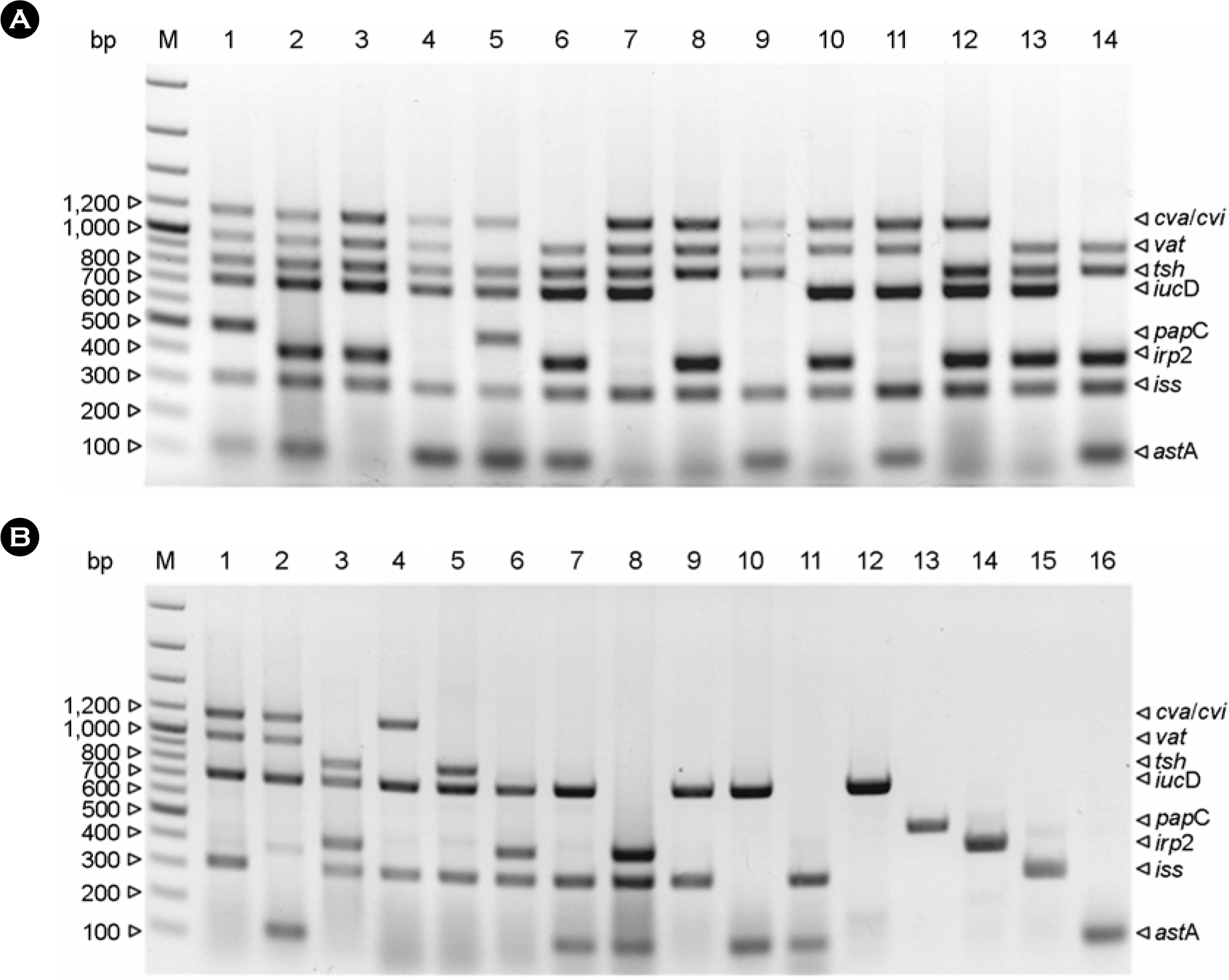
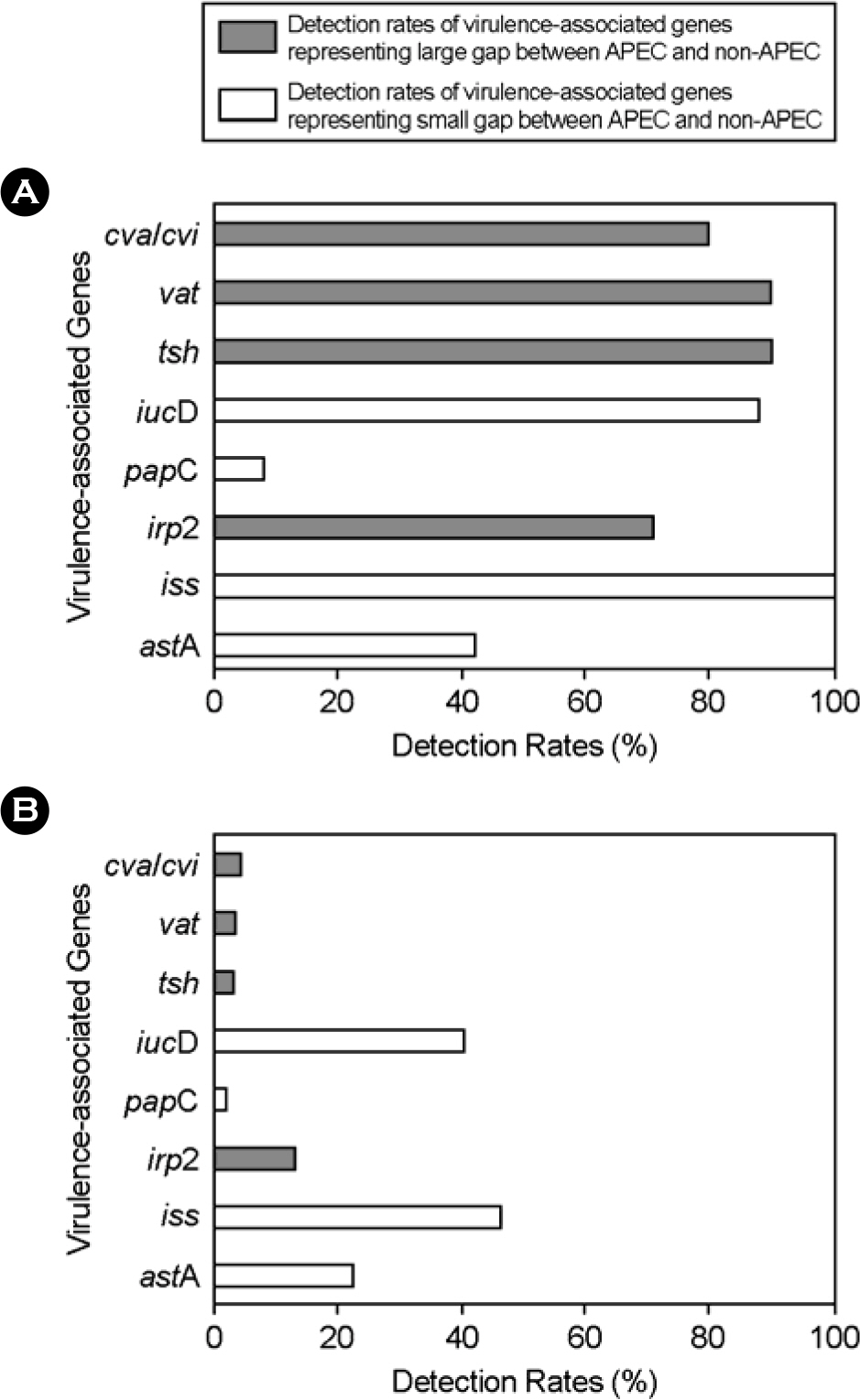
- We examined 216 Escherichia coli (E. coli) isolated from chickens and environmental specimens from hatcheries between 2005 and 2006 in order to evaluate the epidemiological prevalence of avian pathogenic E. coli (APEC) in Korea tentatively by multiplex PCR. The multiplex PCR which was used as tentative criteria of APEC targets 8 virulence-associated genes; enteroaggregative toxin (astA), increased serum survival protein (iss), iron-repressible protein (irp2), P fimbriae (papC), aerobactin (iucD), temperature-sensitive hemagglutinin (tsh), vacuolating autotransporter toxin (vat), and colicin V plasmid operon (cva/cvi) genes. The number of detected genes could be used as a reliable index of their virulence. It was demonstrated that E. coli strains already typed as APEC always harbor 5 to 8 genes, but non-APEC strains harbor less than 4 genes. Assuming the criteria of APEC is a possession of more than 5 virulenceassociated genes, we discriminated 24 APEC strains among the 216 E. coli strains. Contamination rates of APEC in the field were 31.3% in layers, 14.0% in broilers, 2.7% in broiler breeders, and 0.0% in environmental specimens from hatcheries. The combinational tendency of APEC examined is a fundamental possession of astA, iss and iucD genes and addition of cva/cvi, tsh, vat, and irp2 genes which have a critical importance for virulent traits of APEC. Compared with intravenous chicken challenge or embryo lethality assay, multiplex PCR method could be useful to discriminate APEC rapidly for convenient diagnosis.
Keyword
MeSH Terms
Figure
Reference
-
1). Amabile de Campos T., Stehling EG., Ferreira A., Pestana de Castro AF., Brocchi M., Dias da Silveira W. Adhesion properties, fimbrial expression and PCR detection of adhesin-related genes of avian Escherichia coli strains. Vet Microbiol. 106:275–285. 2005.2). Barnes HJ., Nolan LK., Vaillancourt JP. Colibacillosis. pp.p. 691–732. In. Diseases of Poultry. 12th ed. Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. (Ed.),. Blackwell Pub. Professional, Ames, Iowa;2008.3). Dozois CM., Dho-Moulin M., Brée A., Fairbrother JM., Desautels C., Curtiss R 3rd. Relationship between the tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of tsh genetic region. Infect Immun. 68:4145–4154. 2000.4). Dozois CM., Fairbrother JM., Harel J., Bossé M. pap- and pil-related DNA sequences and other virulence determinants associated with Escherichia coli isolated from septicemic chickens and turkeys. Infect Immun. 60:2648–2656. 1992.5). Ellis MG., Arp LH., Lamont SJ. Serum resistance and virulence of Escherichia coli isolated from turkeys. Am J Vet Res. 49:2034–2037. 1988.6). Ewers C., Janssen T., Kiessling S., Philipp HC., Wieler LH. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 49:269–273. 2005.7). Gibbs PS., Maurer JJ., Nolan LK., Wooley RE. Prediction of chicken embryo lethality with the avian Escherichia coli traits complement resistance, colicin V production, and presence of the increased serum survival gene cluster (iss). Avian Dis. 47:370–379. 2003.8). Gibbs PS., Wooley RE. Comparison of the intravenous chicken challenge method with the embryo lethality assay for studies in avian colibacillosis. Avian Dis. 47:672–680. 2003.
Article9). Gilson L., Mahanty HK., Kolter R. Four plasmid genes are required for colicin V synthesis, export, and immunity. J Bacteriol. 169:2466–2470. 1987.
Article10). Gophna U., Oelschlaeger TA., Hacker J., Ron EZ. Yersinia HPI in septicemic Escherichia coli strains isolated from diverse hosts. FEMS Microbiol Lett. 196:57–60. 2001.11). Henderson IR., Navarro-Garcia F., Desvaux M., Fernandez RC., Ala'Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev. 68:692–744. 2004.
Article12). Janben T., Schwarz C., Preikschat P., Voss M., Philipp HC., Wieler LH. Virulence-associated genes in avian pathogenic Escherichia coli (APEC) isolated from internal organs of poultry having died from colibacillosis. Int J Med Microbiol. 291:371–378. 2001.13). Johnson TJ., Siek KE., Johnson SJ., Nolan LK. DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. J Bacteriol. 188:745–758. 2006.14). Kostakioti M., Stathopoulos C. Functional analysis of tsh autotransporter from an avian pathogenic Escherichia coli strain. Infect Immun. 72:5548–5554. 2004.15). Ménard LP., Dubreuil JD. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 (EAST1): a new toxin with an old twist. Crit Rev Microbiol. 28:43–60. 2002.16). Morris M. Poultry health issue. Poultry Times July. 3:11. 1989.17). Paiva de Sousa C., Dubreuil JD. Distribution and expression of the astA gene (EAST1 toxin) in Escherichia coli and Salmonella. Int J Med Microbiol. 291:15–20. 2001.18). Parreira VR., Gyles CL. A novel pathogenicity island integrated adjacent to the thrW tRNA gene of avian pathogenic Escherichia coli encodes a vacuolating autotransporter toxin. Infect Immun. 71:5087–5096. 2003.19). Pfaff-McDonough SJ., Horne SM., Giddings CW., Ebert JO., Doetkott C., Smith MH., Nolan LK. Complement resistance-related traits among Escherichia coli isolates from apparently healthy birds and birds with colibacillosis. Avian Dis. 44:23–33. 2000.20). Provence DL., Curtiss R 3rd. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 62:1369–1380. 1994.21). Rodriguez-Siek KE., Giddings CW., Doetkott C., Johnson TJ., Nolan LK. Characterizing the APEC pathotype. Vet Res. 36:241–256. 2005.
Article22). Salvadori MR., Yano T., Carvalho HE., Parreira VR., Gyles CL. Vacuolating cytotoxin produced by avian pathogenic Escherichia coli. Avian Dis. 45:43–51. 2001.23). Tivendale KA., Allen JL., Ginns CA., Crabb BS., Browning GF. Association of iss and iucA, but not tsh, with plasmid-mediated virulence of avian pathogenic Escherichia coli. Infect Immun. 72:6554–6560. 2004.24). Valvano MA., Crosa JH. Aerobactin iron transport genes commonly encoded by certain ColV plasmids occur in the chromosome of a human invasive strain of Escherichia coli K1. Infect Immun. 46:159–167. 1984.25). Vandekerchove D., Vandemaele F., Adriaensen C., Zaleska M., Hernalsteens JP., De Baets L., Butaye P., Van Immerseel F., Wattiau P., Laevens H., Mast J., Goddeeris B., Pasmans F. Virulence-associated traits in avian Escherichia coli: comparison between isolates from colibacillosis-affected and clinically healthy layer flocks. Vet Microbiol. 108:75–87. 2005.26). Veilleux S., Dubreuil JD. Presence of Escherichia coli carrying the EAST1 toxin gene in farm animals. Vet Res. 37:3–13. 2006.27). Waters VL., Crosa JH. Colicin V virulence plasmids. Microbiol Rev. 55:437–450. 1991.
Article28). Wooley RE., Gibbs PS., Brown TB., Maurer JJ. Chicken embryo lethality assay for determining the virulence of avian Escherichia coli isolates. Avian Dis. 44:318–324. 2000.29). Yamamoto T., Nakazawa M. Detection and sequences of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene in enterotoxigenic E. coli strains isolated from piglets and calves with diarrhea. J Clin Microbiol. 35:223–227. 1997.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation and identification of Escherichia coli O157:H7 using different detection methods and molecular determination by multiplex PCR and RAPD
- Prevalence of Escherichia coli Carrying pks Islands in Bacteremia Patients
- Pathotyping avian pathogenic Escherichia coli strains in Korea
- Detection of Escherichia coli O157 and Escherichia coli O157:H7 by the immunomagnetic separation technique and stx1 and stx2 genes by multiplex PCR in slaughtered cattle in Samsun Province, Turkey
- Detection of 13 Enteric Bacteria and 5 Viruses Causing Acute Infectious Diarrhea Using Multiplex PCR from Direct Stool Specimens