J Vet Sci.
2010 Dec;11(4):321-326. 10.4142/jvs.2010.11.4.321.
Detection of Escherichia coli O157 and Escherichia coli O157:H7 by the immunomagnetic separation technique and stx1 and stx2 genes by multiplex PCR in slaughtered cattle in Samsun Province, Turkey
- Affiliations
-
- 1Food Hygiene and Technology Department, Faculty of Veterinary Medicine, Ondokuz Mayis University, Kurupelit, 55139 Samsun, Turkey. gkhaninat@yahoo.com
- KMID: 1072181
- DOI: http://doi.org/10.4142/jvs.2010.11.4.321
Abstract
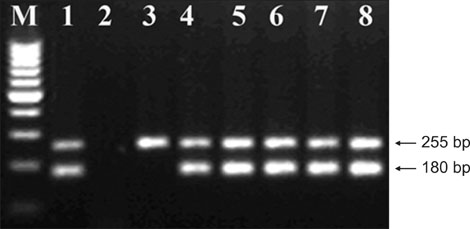
- This study was conducted to investigate the presence of Escherichia (E.) coli O157 and E. coli O157:H7 and stx1 and stx2 genes on cattle carcasses and in rectal samples collected from Samsun Province of Turkey. A total of 200 samples collected from cattle carcasses and the rectal contents of 100 slaughtered cattle from two commercial abattoirs were tested using the immunomagnetic separation technique and multiplex PCR methods. E. coli O157 and E. coli O157:H7 were detected in 52 of the 200 samples (26%) tested. Of the positive samples, 49 were E. coli O157 and three were E. coli O157:H7. The E. coli O157 strain was isolated from 24 carcasses and 25 rectal samples, while E. coli O157:H7 was isolated from two carcasses and one rectal sample. Of the 49 samples positive for E. coli O157, 32 were from the rectal and carcass samples of the same animal, while two E. coli O157:H7 isolates were obtained from rectal swabs and carcasses of the same animal. The stx1 and stx2 genes were both detected in 35 E. coli O157 isolates and one E. coli O157:H7 isolate, but the stx2 gene was only detected alone in two E. coli O157 isolates. Overall, 16 carcasses tested positive for E. coli O157 and one carcass tested positive for E. coli O157:H7 based on both carcass and rectal samples. Overall, the results of this study indicate that cattle carcasses pose a potential risk to human health due to contamination by E. coli O157 and E. coli O157:H7 in the feces.
Keyword
MeSH Terms
Figure
Reference
-
1. Aksoy A, İstanbulluoğlu E, Apan TZ, Yıldırım M, Özarslan B. Kırıkkale ili'nde sığır ve koyun dışkılarında Escherichia coli O157:H7 prevalansının belirlenmesi. Vet Hek Mikrobiol Derg. 2005. 5:3–8.2. Arthur TM, Bosilevac JM, Brichta-Harhay DM, Guerini MN, Kalchayanand N, Shackelford SD, Wheeler TL, Koohmaraie M. Transportation and lairage environment effects on prevalence, numbers, and diversity of Escherichia coli O157:H7 on hides and carcasses of beef cattle at processing. J Food Prot. 2007. 70:280–286.
Article3. Aslantaş Ö, Erdoğan S, Cantekin Z, Gülaçtı İ, Evrendilek GA. Isolation and characterization of verocytotoxin-producing Escherichia coli O157 from Turkish cattle. Int J Food Microbiol. 2006. 106:338–342.
Article4. Barkocy-Gallagher GA, Arthur TM, Rivera-Betancourt M, Nou X, Shackelford SD, Wheeler TL, Koohmaraie M. Seasonal prevalence of Shiga toxin-producing Escherichia coli, including O157:H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. J Food Prot. 2003. 66:1978–1986.
Article5. Carney E, O'Brien SB, Sheridan JJ, Mcdowell DA, Blair IS, Duffy G. Prevalence and level of Escherichia coli O157 on beef trimmings, carcasses and boned head meat at a beef slaughter plant. Food Microbiol. 2006. 23:52–59.
Article6. Centers for Disease Control and Prevention (CDC). Foodborne diseases active surveillance network, 1996. MMWR Morb Mortal Wkly Rep. 1997. 46:258–261.7. Chapman PA, Cerdán Malo AT, Ellin M, Ashton R, Harkin MA. Escherichia coli O157 in cattle and sheep at slaughter, on beef and lamb carcasses and in raw beef and lamb products in South Yorkshire, UK. Int J Food Microbiol. 2001. 64:139–150.
Article8. Chapman PA, Wright DJ, Higgins R. Untreated milk as a source of verotoxigenic E. coli O157. Vet Rec. 1993. 133:171–172.9. Çabalar M, Boynukara B, Gülhan T, Ekin İH. Prevalence of rotavirus, Escherichia coli K99 and O157:H7 in healthy dairy cattle herds in Van, Turkey. Turk J Vet Anim Sci. 2001. 25:191–196.10. de Boer E, Heuvelink AE. Duffy G, Garvey P, McDowell DA, editors. Foods as vehicles of VTEC infection. Verocytotoxigenic E. coli. 2001. 1st ed. Trumbull: Food & Nutrition Press;181–200.
Article11. Eklund M. Enterohemorrhagic Escherichia coli (EHEC) Findings from Humans in Finland. Publications of the National Public Health Institute A. A23. 2005. Helsinki: National Institute for Health and Welfare.12. Elder RO, Keen JE, Siragusa GR, Barkocy-Gallagher GA, Koohmaraie M, Laegreid WW. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc Natl Acad Sci USA. 2000. 97:2999–3003.
Article13. Faith NG, Shere JA, Brosch R, Arnold KW, Ansay SE, Lee MS, Luchansky JB, Kaspar CW. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl Environ Microbiol. 1996. 62:1519–1525.
Article14. Fitzmaurice J. Molecular diagnostic assay for Escherichia coli O157:H7. 2003. Galway: National University of Ireland;Ph.D. Thesis.15. Garber L, Wells S, Schroeder-Tucker L, Ferris K. Factors associated with fecal shedding of verotoxin-producing Escherichia coli O157 on dairy farms. J Food Prot. 1999. 62:307–312.
Article16. Garrido P, Blanco M, Moreno-Paz M, Briones C, Dahbi G, Blanco J, Blanco J, Parro V. STEC-EPEC oligonucleotide microarray: a new tool for typing genetic variants of the LEE pathogenicity island of human and animal Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) strains. Clin Chem. 2006. 52:192–201.
Article17. Gill CO, McGinnis JG. Contamination of beef trimmings with Escherichia coli during a carcass breaking process. Food Res Int. 2000. 33:125–130.
Article18. Greenquist MA, Drouillard JS, Sargeant JM, Depenbusch BE, Shi X, Lechtenberg KF, Nagaraja TG. Comparison of rectoanal mucosal swab cultures and fecal cultures for determining prevalence of Escherichia coli O157:H7 in feedlot cattle. Appl Environ Microbiol. 2005. 71:6431–6433.
Article19. Griffin PM. Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL, editors. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. Infections of the Gastrointestinal Tract. 1995. New York: Raven Press;739–761.20. Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991. 13:60–98.
Article21. Gun H, Yilmaz A, Turker S, Tanlasi A, Yilmaz H. Contamination of bovine carcasses and abattoir environment by Escherichia coli O157:H7 in Istanbul. Int J Food Microbiol. 2003. 84:339–344.
Article22. Guyon R, Dorey F, Malas JP, Grimont F, Foret J, Rouvière B, Collobert JF. Superficial contamination of bovine carcasses by Escherichia coli O157:H7 in a slaughterhouse in Normandy (France). Meat Sci. 2001. 58:329–331.
Article23. Law D. Virulence factors of Escherichia coli O157 and other shiga toxin-producing E. coli. J Appl Microbiol. 2000. 88:729–745.
Article24. Locking ME, O'Brien SJ, Reilly WJ, Wright EM, Campbell DM, Coia JE, Browning LM, Ramsay CN. Risk factors for sporadic cases of Escherichia coli O157 infection: the importance of contact with animal excreta. Epidemiol Infect. 2001. 127:215–220.
Article25. McEvoy JM, Doherty AM, Sheridan JJ, Thomson-Carter FM, Garvey P, Mcguire L, Blair IS, McDowell DA. The prevalence and spread of Escherichia coli O157:H7 at a commercial beef abattoir. J Appl Microbiol. 2003. 95:256–266.
Article26. Mead PS, Griffin PM. Escherichia coli O157:H7. Lancet. 1998. 352:1207–1212.27. Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998. 11:142–201.28. Okrend AJG, Rose BE, Lattuada CP. Isolation of Escherichia coli O157:H7 using O157 specific antibody coated magnetic beads. J Food Prot. 1992. 55:214–217.
Article29. Osek J, Gallien P. Molecular analysis of Escherichia coli O157 strains isolated from cattle and pigs by the use of PCR and pulsed-field gel electrophoresis methods. Vet Med (Praha). 2002. 47:149–158.
Article30. Paton AW, Paton JC. Detection and characterization of shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlya, rfbO111, and rfbO157. J Clin Microbiol. 1998. 36:598–602.31. Philips CA. The epidemiology, detection and control of Escherichia coli O157. J Sci Food Agric. 1999. 79:1367–1381.32. Rasmussen MA, Cray WC Jr, Casey TA, Whipp SC. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol Lett. 1993. 114:79–84.33. Rice DH, Sheng HQ, Wynia SA, Hovde CJ. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguishes Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J Clin Microbiol. 2003. 41:4924–4929.
Article34. Sargeant JM, Gillespie JR, Oberst RD, Phebus RK, Hyatt DR, Bohra LK, Galland JC. Results of a longitudinal study of the prevalence of Escherichia coli O157:H7 on cow-calf farms. Am J Vet Res. 2000. 61:1375–1379.
Article35. Sheng H, Lim JY, Knecht HJ, Li J, Hovde CJ. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect Immun. 2006. 74:4685–4693.
Article36. Slutsker L, Ries AA, Maloney K, Wells JG, Greene KD, Griffin PM. A nationwide case-control study of Escherichia coli O157:H7 infection in the United States. J Infect Dis. 1998. 177:962–966.
Article37. Standing Committee on Agriculture and Resource Management. SCARM Report No. 54. AS 4461: 1997. Australian Standard for Hygienic Production of Meat for Human Consumption. 1997. Melbourne: CSIRO Publishing.38. Tutenel AV, Pierard D, Van Hoof J, Cornelis M, De Zutter L. Isolation and molecular characterization of Escherichia coli O157 isolated from cattle, pigs and chickens at slaughter. Int J Food Microbiol. 2003. 84:63–69.
Article39. Varela-Hernández JJ, Cabrera-Diaz E, Cardona-López MA, Ibarra-Velázquez LM, Rangel-Villalobos H, Castillo A, Torres-Vitela MR, Ramírez-Álvarez A. Isolation and characterization of Shiga toxin-producing Escherichia coli O157:H7 and non-O157 from beef carcasses at a slaughter plant in Mexico. Int J Food Microbiol. 2007. 113:237–241.
Article40. Weagant SD, Bryant JL, Jinneman KG. An improved rapid technique for isolation of Escherichia coli O157:H7 from foods. J Food Prot. 1995. 58:7–12.
Article41. Yilmaz A, Gun H, Ugur M, Turan N, Yilmaz H. Detection and frequency of VT1, VT2 and eaeA genes in Escherichia coli O157 and O157:H7 strains isolated from cattle, cattle carcasses and abattoir environment in Istanbul. Int J Food Microbiol. 2006. 106:213–217.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid Molecular Detection of Escherichia coli O157:H7
- Escherichia coli O157: H7 Infection
- Isolation and identification of Escherichia coli O157:H7 using different detection methods and molecular determination by multiplex PCR and RAPD
- Development of a multiplex loop-mediated isothermal amplification assay to detect shiga toxin-producing Escherichia coli in cattle
- All blood, No stool: enterohemorrhagic Escherichia coli O157:H7 infection