Korean J Radiol.
2013 Apr;14(2):307-315. 10.3348/kjr.2013.14.2.307.
Serial MR Analysis of Early Permanent and Transient Ischemia in Rats: Diffusion Tensor Imaging and High b Value Diffusion Weighted Imaging
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul 110-744, Korea. neurorad63@gmail.com
- 2Human Medical Imaging & Intervention Center, Seoul 137-902, Korea.
- 3Department of Radiology, Soonchunhyang University Bucheon Hospital, Bucheon 420-767, Korea.
- 4Department of Radiology, Korea University Hospital, Seoul 135-705, Korea.
- KMID: 1482792
- DOI: http://doi.org/10.3348/kjr.2013.14.2.307
Abstract
OBJECTIVE
To evaluate the temporal evolution and diagnostic values of the diffusion tensor imaging (DTI) and the high b value diffusion weighted imaging (DWI) in the early permanent and transient cerebral ischemia.
MATERIALS AND METHODS
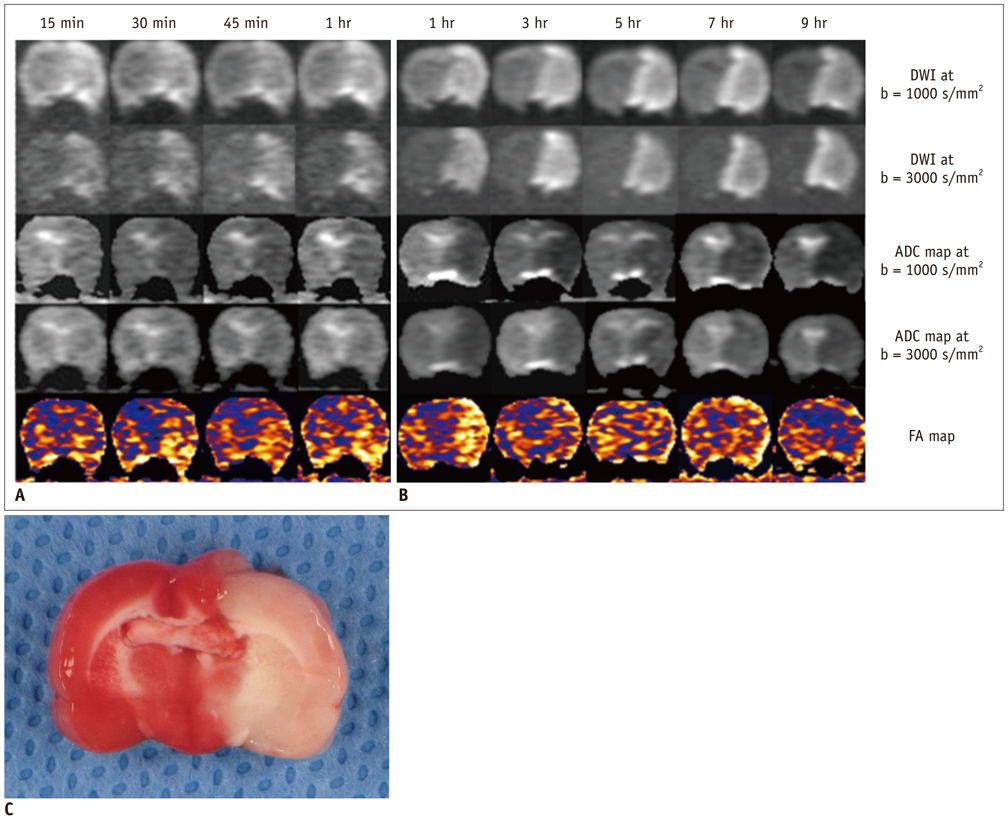
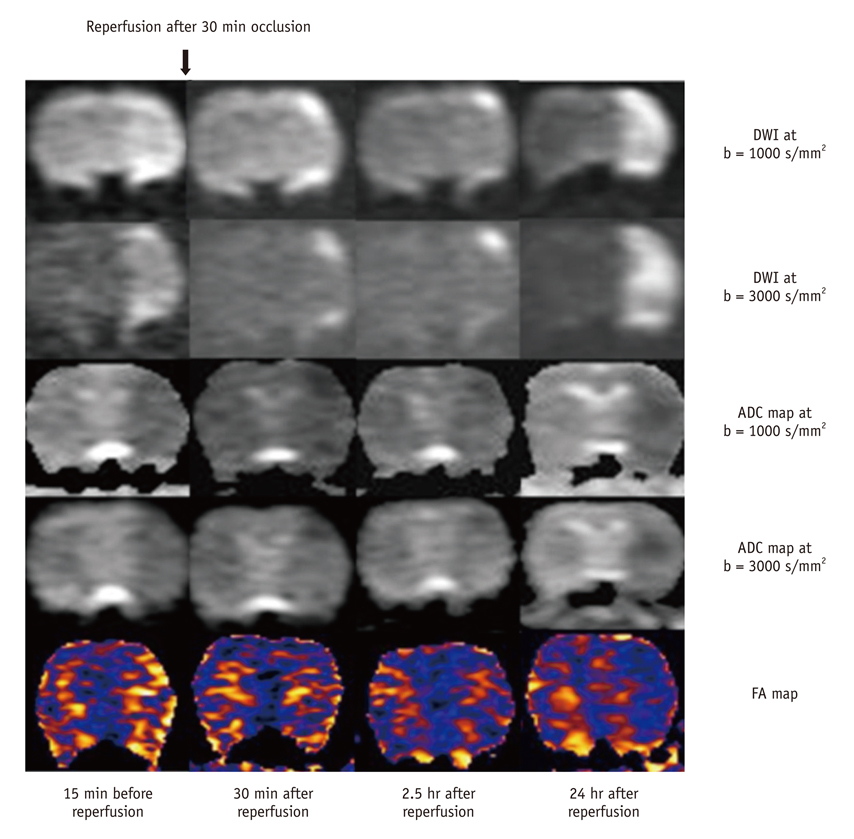
For permanent or 30-minute transient-ischemia induced 30 rats, DTI and DWIs at both high b (b = 3000 s/mm2) and standard b value (b = 1000 s/mm2) were obtained at the following conditions: at 15, 30, 45, 60 minutes after the occlusion of what for hyperacute permanent ischemia; at 1, 3, 5, 7, 9 hours after the occlusion for acute permanent ischemia; and at 15 minutes before reperfusion, 0.5, 2.5, and 24 hours after reperfusion for transient ischemia. The diffusion parameters and their ratios were obtained and compared between different b values, and among different time points and groups, respectively.
RESULTS
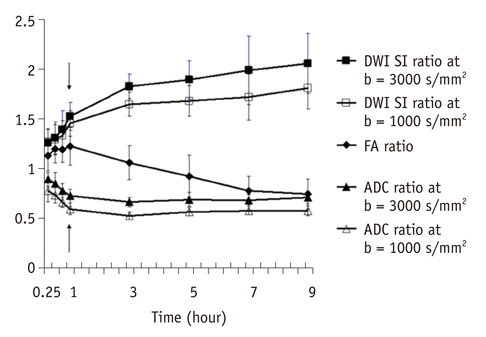
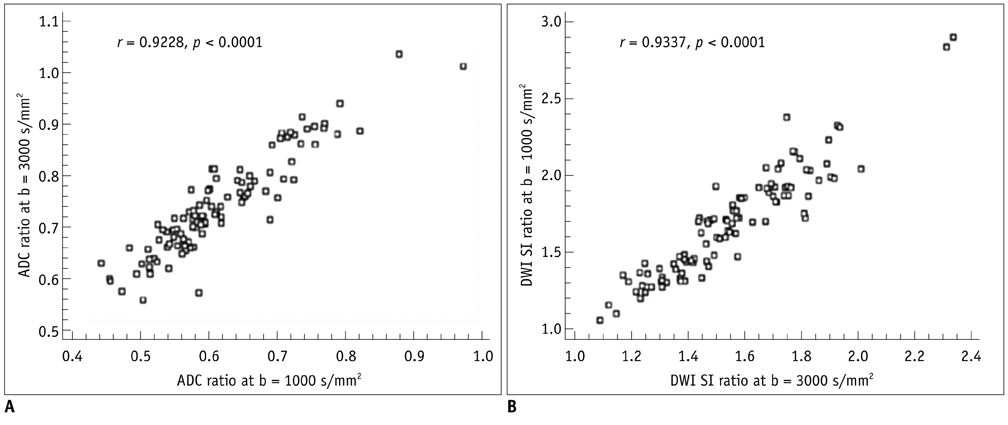
For both b values, the apparent diffusion coefficient (ADC) ratio decreased for first three hours, and then slightly increased until 9 hours after the occlusion during a gradual continuous increase of DWI signal intensity (SI) ratio, with excellent correlation between ADC ratios and DWI SI ratios. The DWI showed a higher contrast ratio, but the ADC map showed a lower contrast ratio for permanent ischemia at high b value than at standard b value. Fractional anisotropy (FA) increased for 1 hour, then gradually decreased until 9 hours after the occlusion in permanent ischemia and showed transient normalization and secondary decay along with change in ADC in transient ischemia.
CONCLUSION
This study presents characteristic initial elevation and secondary decay of FA, higher contrast ratio of DWI, and lower contrast ratio of ADC map at high b value, in addition to the time evolutions of diffusion parameters in early permanent and transient ischemia.
Keyword
MeSH Terms
Figure
Reference
-
1. DeLano MC, Cooper TG, Siebert JE, Potchen MJ, Kuppusamy K. High-b-value diffusion-weighted MR imaging of adult brain: image contrast and apparent diffusion coefficient map features. AJNR Am J Neuroradiol. 2000. 21:1830–1836.2. Niendorf T, Dijkhuizen RM, Norris DG, van Lookeren Campagne M, Nicolay K. Biexponential diffusion attenuation in various states of brain tissue: implications for diffusion-weighted imaging. Magn Reson Med. 1996. 36:847–857.3. Toyoda K, Kitai S, Ida M, Suga S, Aoyagi Y, Fukuda K. Usefulness of high-b-value diffusion-weighted imaging in acute cerebral infarction. Eur Radiol. 2007. 17:1212–1220.4. Seo HS, Chang KH, Na DG, Kwon BJ, Lee DH. High b-value diffusion (b = 3000 s/mm2) MR imaging in cerebral gliomas at 3T: visual and quantitative comparisons with b = 1000 s/mm2. AJNR Am J Neuroradiol. 2008. 29:458–463.5. Meyer JR, Gutierrez A, Mock B, Hebron D, Prager JM, Gorey MT, et al. High-b-value diffusion-weighted MR imaging of suspected brain infarction. AJNR Am J Neuroradiol. 2000. 21:1821–1829.6. Burdette JH, Elster AD. Diffusion-weighted imaging of cerebral infarctions: are higher B values better? J Comput Assist Tomogr. 2002. 26:622–627.7. Kim HJ, Choi CG, Lee DH, Lee JH, Kim SJ, Suh DC. High-b-value diffusion-weighted MR imaging of hyperacute ischemic stroke at 1.5T. AJNR Am J Neuroradiol. 2005. 26:208–215.8. Melhem ER, Mori S, Mukundan G, Kraut MA, Pomper MG, van Zijl PC. Diffusion tensor MR imaging of the brain and white matter tractography. AJR Am J Roentgenol. 2002. 178:3–16.9. Ahn S, Lee SK. Diffusion tensor imaging: exploring the motor networks and clinical applications. Korean J Radiol. 2011. 12:651–661.10. Tong T, Zhenwei Y, Xiaoyuan F. Transient ischemic attack and stroke can be differentiated by analyzing the diffusion tensor imaging. Korean J Radiol. 2011. 12:280–288.11. Yang Q, Tress BM, Barber PA, Desmond PM, Darby DG, Gerraty RP, et al. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke. 1999. 30:2382–2390.12. Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999. 212:785–792.13. Zelaya F, Flood N, Chalk JB, Wang D, Doddrell DM, Strugnell W, et al. An evaluation of the time dependence of the anisotropy of the water diffusion tensor in acute human ischemia. Magn Reson Imaging. 1999. 17:331–348.14. Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000. 31:1965–1972. discussion 1972-1973.15. Li F, Han S, Tatlisumak T, Carano RA, Irie K, Sotak CH, et al. A new method to improve in-bore middle cerebral artery occlusion in rats: demonstration with diffusion- and perfusion-weighted imaging. Stroke. 1998. 29:1715–1719. discussion 1719-1720.16. Gerriets T, Li F, Silva MD, Meng X, Brevard M, Sotak CH, et al. The macrosphere model: evaluation of a new stroke model for permanent middle cerebral artery occlusion in rats. J Neurosci Methods. 2003. 122:201–211.17. Bardutzky J, Shen Q, Henninger N, Bouley J, Duong TQ, Fisher M. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke. 2005. 36:2000–2005.18. Dijkhuizen RM, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Regional assessment of tissue oxygenation and the temporal evolution of hemodynamic parameters and water diffusion during acute focal ischemia in rat brain. Brain Res. 1997. 750:161–170.19. Brugières P, Thomas P, Maraval A, Hosseini H, Combes C, Chafiq A, et al. Water diffusion compartmentation at high b values in ischemic human brain. AJNR Am J Neuroradiol. 2004. 25:692–698.20. Burdette JH, Elster AD, Ricci PE. Acute cerebral infarction: quantification of spin-density and T2 shine-through phenomena on diffusion-weighted MR images. Radiology. 1999. 212:333–339.21. Liu Y, D'Arceuil HE, Westmoreland S, He J, Duggan M, Gonzalez RG, et al. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke. 2007. 38:138–145.22. Bae MS, Jahng GH, Ryu CW, Kim EJ, Choi WS, Yang DM. Effect of intravenous gadolinium-DTPA on diffusion tensor MR imaging for the evaluation of brain tumors. Neuroradiology. 2009. 51:793–802.23. Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002. 52:698–703.24. Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003. 34:2729–2735.25. Chen F, Suzuki Y, Nagai N, Peeters R, Coenegrachts K, Coudyzer W, et al. Visualization of stroke with clinical MR imagers in rats: a feasibility study. Radiology. 2004. 233:905–911.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Tensor Imaging: Exploring the Motor Networks and Clinical Applications
- Principle and Experiments in Diffusion Tensor Imaging
- Brain Diffusion Tensor MR Imaging
- MR Imaging of Skeletal Muscle Injury in Rabbit: Comparison bet ween Diffusion and T2-weighted MR Images
- Delayed Anoxic Encephalopathy after Carbon Monoxide Poisoning: Evaluation of Therapeutic Effect by Serial Diffusion-Tensor Magnetic Resonance Imaging and Neurocognitive Test