Tuberc Respir Dis.
2008 Jul;65(1):7-14. 10.4046/trd.2008.65.1.7.
The Relation of Residual Pleural Thickening with Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases of Pleural Effusion in Patients with Tuberculous Pleuritis
- Affiliations
-
- 1Graduate School of Medicine, Gachon University of Medicine and Science, Incheon, Korea.
- 2Division of Pulmonology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea. anch@gilhospital.com
- KMID: 1478210
- DOI: http://doi.org/10.4046/trd.2008.65.1.7
Abstract
-
BACKGROUND: Residual pleural thickening (RPT) is the most frequent complication of tuberculous pleurisy (TP), and this can happen despite of administering adequate anti-tuberculous (TB) therapy. Yet there was no definite relation between RPT and other variables. The aim of this study was to examine matrix metalloproteinases (MMPs) and the inhibitors of metalloproteinases (TIMPs) and to identify the factors that can predict the occurrence of RPT.
METHODS
The patients with newly-detected pleural effusions were prospectively enrolled in this study from January 2004 to June 2005. The levels of MMP-1, -2, -8 and -9, and TIMP-1 and -2 were determined in the serum and pleural fluid by ELISA. The residual pleural thickness was measured at the completion of treatment and at the point of the final follow-up with the chest X-ray films.
RESULTS
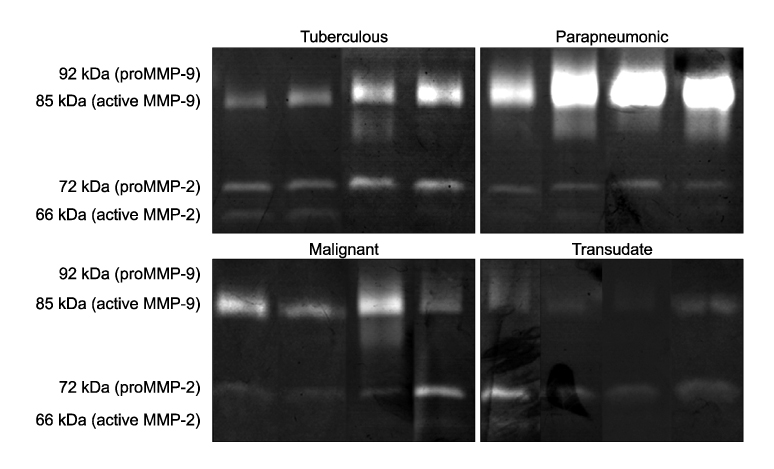
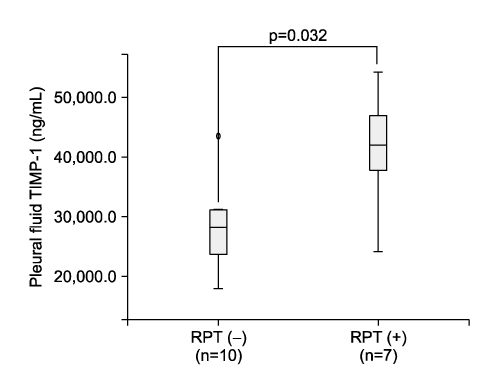
The study included 39 patients with pleural fluid (PF). Twenty-three had tuberculous effusion, 7 had parapneumonic effusion, 7 had malignant effusion and 2 had transudates. For the 17 patients who completed the anti-TB treatment among the 23 patients with TP, 7 (41%) had RPT and 10 (59%) did not. The level of PF TIMP-1 in the patients with RPT (41,405.9+/-9,737.3 ng/mL) was significantly higher than that of those patients without RPT (29,134.9+/-8,801.8) at the completion of treatment (p=0.032). In 13 patients who were followed-up until a mean of 8+/-5 months after treatment, 2 (15%) had RPT and 11 (85%) did not. The level of PF TIMP-2 in the patients with RPT (34.4+/-6.5 ng/mL) was lower than that of those patients without RPT (44.4+/-15.5) at the point of the final follow-up (p=0.038).
CONCLUSION
The residual pleural thickening in TP might be related to the TIMP-1 and TIMP-2 levels in the pleural fluid.
Keyword
MeSH Terms
-
Enzyme-Linked Immunosorbent Assay
Exudates and Transudates
Follow-Up Studies
Humans
Matrix Metalloproteinases
Metalloproteases
Pleural Effusion
Pleurisy
Prospective Studies
Thorax
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Tuberculosis, Pleural
X-Ray Film
Matrix Metalloproteinases
Metalloproteases
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Figure
Reference
-
1. Eickelberg O, Sommerfeld CO, Wyser C, Tamm M, Reichenberger F, Bardin PG, et al. MMP and TIMP expression pattern in pleural effusions of different origins. Am J Respir Crit Care Med. 1997. 156:1987–1992.2. Hoheisel G, Sack U, Hui DSC, Huse K, Chan KS, Chan KK, et al. Occurrence of matrix metalloproteinases and tissue inhibitors of metalloproteinases in tuberculous pleuritis. Tuberculosis. 2001. 81:203–209.3. Jin HY, Lee KS, Jin SM, Lee YC. Vascular endothelial growth factor correlates with matrix metalloproteinase-9 in the pleural effusion. Respir Med. 2004. 98:115–122.4. Kotyza J, Pesek M, Puzman P, Havel D. Progelatinase B/matrix metalloproteinase-9 proenzyme as a marker of pleural inflammation. Exp Lung Res. 2004. 30:297–309.5. Park KJ, Hwang SC, Sheen SS, Oh YJ, Han JH, Lee KB. Expression of matrix metalloproteinase-9 in pleural effusions of tuberculosis and lung cancer. Respiration. 2005. 72:166–175.6. Iglesias D, Alegre J, Aleman C, Ruiz E, Soriano T, Armadans LI, et al. Metalloproteinaes and tissue inhibitors of metalloproteinase in exudative pleural effusions. Eur Respir J. 2005. 25:104–109.7. Di Carlo A, Terracciano D, Mariano A, Macchia V. Matrix metalloproteinase-2 and matrix metalloproteinase-9 type IV collagenases in serum of patients with pleural effusions. Int J Oncol. 2005. 26:1363–1368.8. Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusion: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972. 77:507–513.9. Ocaña I, Martínez Vázquez JM, Segura RM, Fernandez de Sevilla T, Capdevila JA. Adenosine deaminase in pleural fluid: Test for diagnosis of tuberculous pleural effusion. Chest. 1983. 84:51–53.10. Light RW, Girad WM, Jenkinson SG, George RB. Parapneumonic effusions. Am J Med. 1980. 69:507–512.11. Lee BH, Jee HS, Choi JC, Park YB, An CH, Kim JY, et al. Therapeutic effect of prednisolone in tuberculous pleurisy: a prospective study for the prevention of the pleural adhesion. Tuberc Respir Dis. 1999. 46:481–488.12. Kyung SY, Kim YJ, Lim YH, AN CH, Lee SP, Park JW, et al. Spontaneous resolution of residual pleural thickening in tuberculous pleurisy. Tuberc Repir Dis. 2005. 59:69–76.13. Kim YJ, Kim JH, Jeon HK, Kim MK, Jo YC, Kyung SY, et al. The expression of MMPs and TIMPs in IPF and NSIP. Tuberc Repir Dis. 2006. 61:447–455.14. Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999. 274:21491–21494.15. Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992. 7:120–125.16. Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN. Effect of Mycobacterioum tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996. 51:306–311.17. O'Connell JP, Willenbrock F, Docherty AJ, Eaton D, Murphy G. Analysis of the role of the COOH-terminal domain in the activation, proteolytic activity, and tissue inhibitor of metalloproteinase interactions of gelatinase B. J Biol Chem. 1994. 269:14967–14973.18. Light RW, Lee YCG. Textbook of pleural diseases. 2003. 1st ed. New York: Oxford University Press Inc..19. Cho JH, Nam JH, Lee KH, Yoon BK, Ryu JS, Kwak SM, et al. Matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 levels in exudative pleural effusions. Tuberc Respir Dis. 2005. 59:517–521.20. Howard EW, Bullen EC, Banda MJ. Preferential inhibition of 72- and 92-kDa gelatinases by tissue inhibitor of metalloproteinases-2. J Biol Chem. 1991. 266:13070–13075.21. Baragi VM, Fliszar CJ, Conroy MC, Ye QZ, Shipley JM, Welgus HG. Contribution of the C-terminal domain of metalloproteinases to binding by tissue inhibitor of metalloproteinases. C-terminal truncated stromelysin and matrilysin exhibit equally compromised binding affinities as compared to full-length stromelysin. J Biol Chem. 1994. 269:2692–2697.22. Nguyen Q, Willenbrock F, Cockett MI, O'Shea M, Docherty AJ, Murphy G. Different domain interactions are involved in the binding of tissue inhibitors of metalloproteinases to stromelysin-1 and gelatinase A. Biochemistry. 1994. 33:2089–2095.23. Stetler-Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989. 264:17374–17378.24. Chesler L, Golde DW, Bersch N, Johnson MD. Metalloproteinase inhibition and erythroid potentiation are independent activities of tissue inhibitor of metalloproteinases-1. Blood. 1995. 86:4506–4515.25. Kinoshita T, Sato H, Okada A, Ohuchi E, Imai K, Okada Y, et al. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J Biol Chem. 1998. 273:16098–16103.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of Tuberculous Pleuritis
- Factors Associated with Residual Pleural Thickening After Chemotherapy in Tyberculous Pleurisy
- Clinical Significance of Tissue Levels of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Gastric Cancer
- The Utility of Pleural Adenosine Deaminase for Diagnosis of Differentiating Tuberculous Pleural Effusion in Children
- Tuberculous Pleural Effusion vs Empyema: It is Possible to Differentiate Based on CT Findings?