Immune Netw.
2009 Apr;9(2):53-57. 10.4110/in.2009.9.2.53.
Transmembrane Adaptor Proteins Positively Regulating the Activation of Lymphocytes
- Affiliations
-
- 1Department of Life Science, Ewha Womans' University, Seoul, Korea. YUNYUNG@ewha.ac.kr
- KMID: 1474595
- DOI: http://doi.org/10.4110/in.2009.9.2.53
Abstract
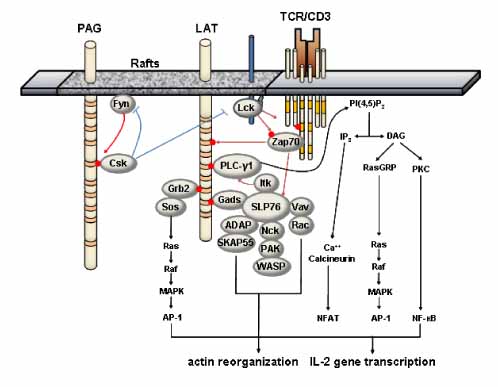
- Engagement of the immunoreceptors initiates signaling cascades resulting in lymphocyte activation and differentiation to effector cells, which are essential for the elimination of pathogens from the body. For the transduction of these immunoreceptor-mediated signals, several linker proteins termed transmembrane adaptor proteins (TRAPs) were shown to be required. TRAPs serve as platforms for the assembly and membrane targeting of the specific signaling proteins. Among seven TRAPs identified so far, LAT and LIME were shown to act as a positive regulator in TCR-mediated signaling pathways. In this review, we will discuss the functions of LAT and LIME in modulating T cell development, activation and differentiation.
Keyword
MeSH Terms
Figure
Reference
-
1. Latour S, Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol. 2001. 13:299–306.
Article2. Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998. 92:83–92.3. Hur EM, Son M, Lee OH, Choi YB, Park C, Lee H, Yun Y. LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation. J Exp Med. 2003. 198:1463–1473.
Article4. Brdicková N, Brdicka T, Angelisová P, Horváth O, Spicka J, Hilgert I, Paces J, Simeoni L, Kliche S, Merten C, Schraven B, Horejsí V. LIME: a new membrane Raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. J Exp Med. 2003. 198:1453–1462.5. Zhu M, Janssen E, Leung K, Zhang W. Molecular cloning of a novel gene encoding a membrane-associated adaptor protein (LAX) in lymphocyte signaling. J Biol Chem. 2002. 277:46151–46158.
Article6. Brdicka T, Imrich M, Angelisová P, Brdicková N, Horváth O, Spicka J, Hilgert I, Lusková P, Dráber P, Novák P, Engels N, Wienands J, Simeoni L, Osterreicher J, Aguado E, Malissen M, Schraven B, Horejsí V. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002. 196:1617–1626.7. Brdicka T, Pavlistoví D, Leo A, Bruyns E, Korinek V, Angelisová P, Scherer J, Shevchenko A, Hilgert I, Cerný J, Drbal K, Kuramitsu Y, Kornacker B, Horejsí V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000. 191:1591–1604.
Article8. Pfrepper KI, Marie-Cardine A, Simeoni L, Kuramitsu Y, Leo A, Spicka J, Hilgert I, Scherer J, Schraven B. Structural and functional dissection of the cytoplasmic domain of the transmembrane adaptor protein SIT (SHP2-interacting transmembrane adaptor protein). Eur J Immunol. 2001. 31:1825–1836.
Article9. Bruyns E, Marie-Cardine A, Kirchgessner H, Sagolla K, Shevchenko A, Mann M, Autschbach F, Bensussan A, Meuer S, Schraven B. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR-CD3-zeta complex, recruits intracellular signaling proteins to the plasma membrane. J Exp Med. 1998. 188:561–575.
Article10. Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006. 359:re14.
Article11. Michel F, Attal-Bonnefoy G, Mangino G, Mise-Omata S, Acuto O. CD28 as a molecular amplifier extending TCR ligation and signaling capabilities. Immunity. 2001. 15:935–945.
Article12. Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998. 9:239–246.13. Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000. 275:23355–23361.
Article14. Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998. 9:617–626.
Article15. Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999. 11:943–950.
Article16. Boerth NJ, Sadler JJ, Bauer DE, Clements JL, Gheith SM, Koretzky GA. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J Exp Med. 2000. 192:1047–1058.
Article17. Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999. 10:323–332.
Article18. Sommers CL, Menon RK, Grinberg A, Zhang W, Samelson LE, Love PE. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. J Exp Med. 2001. 194:135–142.
Article19. Aguado E, Richelme S, Nuñez-Cruz S, Miazek A, Mura AM, Richelme M, Guo XJ, Sainty D, He HT, Malissen B, Malissen M. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002. 296:2036–2040.
Article20. Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacaná E, Menon RK, Shores EW, Samelson LE, Love PE. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002. 296:2040–2043.
Article21. Zhu M, Shen S, Liu Y, Granillo O, Zhang W. Cutting Edge: localization of linker for activation of T cells to lipid rafts is not essential in T cell activation and development. J Immunol. 2005. 174:31–35.
Article22. Hundt M, Tabata H, Jeon MS, Hayashi K, Tanaka Y, Krishna R, De Giorgio L, Liu YC, Fukata M, Altman A. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 2006. 24:513–522.
Article23. Grégoire C, Simova S, Wang Y, Sansoni A, Richelme S, Schmidt-Giese A, Simeoni L, Angelisova P, Reinhold D, Schraven B, Horejsi V, Malissen B, Malissen M. Deletion of the LIME adaptor protein minimally affects T and B cell development and function. Eur J Immunol. 2007. 37:3259–3269.
Article24. Ahn E, Lee H, Yun Y. LIME acts as a transmembrane adapter mediating BCR-dependent B-cell activation. Blood. 2006. 107:1521–1527.
Article25. Tedoldi S, Paterson JC, Hansmann ML, Natkunam Y, Rüdiger T, Angelisova P, Du MQ, Roberton H, Roncador G, Sanchez L, Pozzobon M, Masir N, Barry R, Pileri S, Mason DY, Marafioti T, Horejsi V. Transmembrane adaptor molecules: a new category of lymphoid-cell markers. Blood. 2006. 107:213–221.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interaction of FcalphaR with gamma Subunit of FcgammaRIalpha to Modulate Cbl, Shc and Grb2 Adaptor Proteins
- Specific kinesin and dynein molecules participate in the unconventional protein secretion of transmembrane proteins
- Functional proteomics, human genetics and cancer biology of GIPC family members
- ASK1 is Involved in EBV LMP1-induced NF-kappaB Activation
- Evolutionary Signature of Information Transfer Complexity in Cellular Membrane Proteomes