Immune Netw.
2009 Oct;9(5):203-207. 10.4110/in.2009.9.5.203.
Production of Monoclonal Antibodies Specific to FimA of Porphyromonas gingivalis and Their Inhibitory Activity on Bacterial Binding
- Affiliations
-
- 1Division of Biological Sciences and The Institute for Molecular Biology and Genetics, Chonbuk National University, Jeonju 561-756, Korea. tgkim@jbnu.ac.kr
- 2Jeonju Biomaterials Institute, Jeonju 561-300, Korea.
- 3Department of Dental Microbiology, Kyunghee University Dental School, Seoul 130-701, Korea.
- KMID: 1474581
- DOI: http://doi.org/10.4110/in.2009.9.5.203
Abstract
- BACKGROUND
The FimA of Porphyromonas gingivalis is a crucial pathogenic component of the bacteria and has been implicated as a target for vaccine development against the periodontal diseases.
METHODS
In this study, the purified fimbriae (FimA subunit polymers) protein was used for immunization in their native form and B hybridoma clones producing antibodies specific to FimA were established.
RESULTS
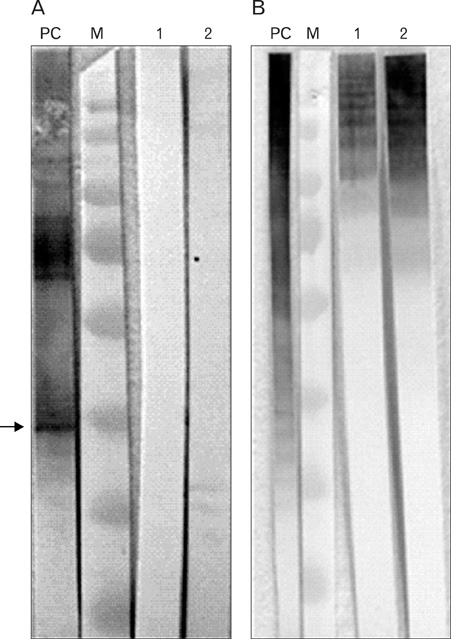
The monoclonal antibodies prepared from selected two clones, designated #123 (IgG2b/ kappa) and #265 (IgG1/kappa), displayed different patterns of binding activity against the cognate antigen. Both antibodies reacted with conformational epitopes expressed by partially dissociated oligomers, but not with monomer as elucidated by Western blot analysis. Ascites fluid containing the monoclonal antibodies showed the inhibitory activity against P. gingivalis to saliva-coated hydroxyapatite beads, an in vitro model for the pellicle-coated tooth surface.
CONCLUSION
These results suggest that the monoclonal antibodies could be used as vaccine material against the periodontal diseases through passive immunization.
MeSH Terms
Figure
Reference
-
1. Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J Dent Res. 2006. 85:392–403.
Article2. Griffen AL, Becker MR, Lyons SR, Moeschberger ML, Leys EJ. Prevalence of porphyromonas gingivalis and periodontal health status. J Clin Microbiol. 1998. 36:3239–3242.
Article3. Amano A. Molecular interaction of Porphyromonas gingivalis with host cells: implication for the microbial pathogenesis of periodontal disease. J Periodontol. 2003. 74:90–96.
Article4. Ezzo PJ, Cutler CW. Microorganisms as risk indicators for periodontal disease. Periodontol 2000. 2003. 32:24–35.
Article5. Shibata Y, Hosogi Y, Hayakawa M, Hori N, Kamada M, Abiko Y. Construction of novel human monoclonal antibodies neutralizing Porphyromonas gingivalis hemagglutination activity using transgenic mice expressing human Ig loci. Vaccine. 2005. 23:3850–3856.
Article6. Jotwani R, Cutler CW. Fimbriated porphyromonas gingivalis is more efficient than fimbria-deficient P. gingivalis in entering human dendritic cells in vitro and induces an inflammatory Th1 effector response. Infect Immun. 2004. 72:1725–1732.
Article7. Sharma A, Honma K, Evans RT, Hruby DE, Genco RJ. Oral immunization with recombinant streptococcus gordonii expressing porphyromonas gingivalis FimA domains. Infect Immun. 2001. 69:2928–2934.
Article8. Shin EA, Lee JY, Kim TG, Park YK, Langridge WH. Synthesis and assembly of an adjuvanted Porphyromonas gingivalis fimbrial antigen fusion protein in plants. Protein Expr Purif. 2006. 47:99–109.
Article9. Takahashi Y, Kumada H, Hamada N, Haishima Y, Ozono S, Isaka M, Yasuda Y, Tochikubo K, Umemoto T. Induction of immune responses and prevention of alveolar bone loss by intranasal administration of mice with Porphyromonas gingivalis fimbriae and recombinant cholera toxin B subunit. Oral Microbiol Immunol. 2007. 22:374–380.
Article10. Evans RT, Klausen B, Genco RJ. Immunization with fimbrial protein and peptide protects against Porphyromonas gingivalis-induced periodontal tissue destruction. Adv Exp Med Biol. 1992. 327:255–262.
Article11. Evans RT, Klausen B, Sojar HT, Bedi GS, Sfintescu C, Ramamurthy NS, Golub LM, Genco RJ. Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infect Immun. 1992. 60:2926–2935.
Article12. Hamada N, Watanabe K, Sasakawa C, Yoshikawa M, Yoshimura F, Umemoto T. Construction and characterization of a fimA mutant of Porphyromonas gingivalis. Infect Immun. 1994. 62:1696–1704.
Article13. Isogai H, Isogai E, Yoshimura F, Suzuki T, Kagota W, Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988. 33:479–485.
Article14. Casadevall A. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis. 2002. 8:833–841.
Article15. Ma JK, Hunjan M, Smith R, Kelly C, Lehner T. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. Infect Immun. 1990. 58:3407–3414.
Article16. Lee JY, Sojar HT, Amano A, Genco RJ. Purification of major fimbrial proteins of Porphyromonas gingivalis. Protein Expr Purif. 1995. 6:496–500.
Article17. Lee JY, Sojar HT, Bedi GS, Genco RJ. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992. 60:1662–1670.
Article18. Kirakodu SS, Govindaswami M, Novak MJ, Ebersole JL, Novak KF. Optimizing qPCR for the quantification of periodontal pathogens in a complex plaque biofilm. Open Dent J. 2008. 2:49–55.
Article19. Hosogi Y, Duncan MJ. Gene expression in Porphyromonas gingivalis after contact with human epithelial cells. Infect Immun. 2005. 73:2327–2335.
Article20. Dickinson DP, Kubiniec MA, Yoshimura F, Genco RJ. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988. 170:1658–1665.
Article21. Ito HO, Nakashima T, So T, Hirata M, Inoue M. Immunodominance of conformation-dependent B-cell epitopes of protein antigens. Biochem Biophys Res Commun. 2003. 308:770–776.
Article22. Yoshimura F, Sugano T, Kawanami M, Kato H, Suzuki T. Detection of specific antibodies against fimbriae and membrane proteins from the oral anaerobe Bacteroides gingivalis in patients with periodontal diseases. Microbiol Immunol. 1987. 31:935–941.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of fimA Genotypes of Porphyromonas gingivalis Strains in peri-implant sulcus
- Development of monoclonal antibody against Porphyromonas gingivalis heat shock protein
- Prevalence of fimA Genotypes of Porphyromonas gingivalis Strains in peri-implantitis patients
- Identification of mono- or poly-specific monoclonal antibody to Porphyromonas gingivalis heat-shock protein 60
- Fusobacterium nucleatum modulates serum binding to Porphyromonas gingivalis biofilm