J Bacteriol Virol.
2009 Jun;39(2):103-112. 10.4167/jbv.2009.39.2.103.
The Bacterial Surface Expression of SARS Viral Epitope using Salmonella typhi Cytolysin A
- Affiliations
-
- 1Clinical Vaccine R&D Center and Department of Microbiology, Chonnam National University Medical School, Gwangju, Korea. yjhong@chonnam.ac.kr
- 2International Vaccine Institute, Seoul, Korea.
- 3Sun Moon University, A-San, Korea.
- KMID: 1474170
- DOI: http://doi.org/10.4167/jbv.2009.39.2.103
Abstract
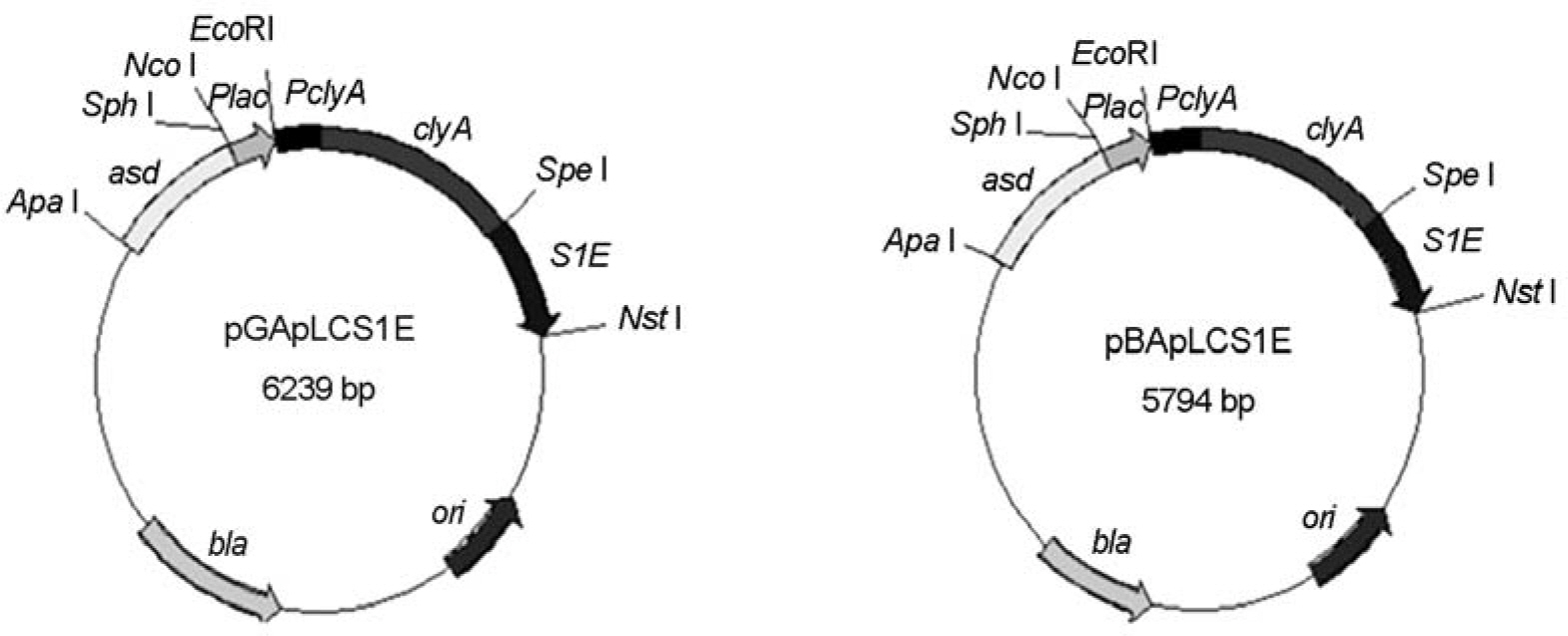
- The cytolysin A (ClyA) is a 34 kDa pore-forming cytotoxic protein and expressed by some enteric bacteria including Salmonella typhi. This toxin is transported on the bacterial surface and secreted without posttranslational modification. Using the surface display of ClyA, the expression vectors for 193-aa immunogenic antigen of spike protein (termed S1E) from severe acute respiratory syndrome coronavirus (SARS-CoV) were constructed. The vectors carried a gene encoding S. typhi ClyA conjugated to S1E at the C terminus (termed ClyA-S1E) and asd gene in pGEM-T and pBR322, named pGApLCS1E and pBApLCS1E, respectively. An asd-mutated E. coli transformed with these vectors could grow without diaminopimelic acid (DAP), indicating that they were stably maintained in such mutants. ClyA-S1E recombinant proteins from these vectors were expressed on the surface of the attenuated S. typhimurium deficient of global virulence gene regulator, ppGpp. However, they did not show the hemolytic activity on the blood agar plate and cytotoxicity against HeLa cells. To examine whether bacteria expressing ClyA-S1E induced the immune response against S1E, S. typhimurium deficient of ppGpp and Asd was transformed with these vectors and orally immunized in mice. In the western blotting against GST-conjugated S1E using the immunized mouse sera, it was shown that the significant band was detected in the mouse serum by the bacteria transformed with pGApLCS1E but not with pBApLCS1E. It indicates that the immune response producing antibody was dependent on the expression level of ClyA-S1E. Therefore, ClyA delivery system can be used for SARS vaccine development.
Keyword
MeSH Terms
Figure
Reference
-
1). Garmory HS., Brown KA., Titball RW. Salmonella vaccines for use in humans: present and future perspectives. FEMS Microbiol Rev. 2002. 26:339–53.2). Garmory HS., Leary SE., Griffin KF., Williamson ED., Brown KA., Titball RW. The use of live attenuated bacteria as a delivery system for heterologous antigens. J Drug Target. 2003. 11:471–9.
Article3). Kochi SK., Killeen KP., Ryan US. Advances in the development of bacterial vector technology. Expert Rev Vaccines. 2003. 2:31–43.4). Lee JS., Shin KS., Pan JG., Kim CJ. Surface-displayed viral antigens on Salmonella carrier vaccine. Nat Biotechnol. 2000. 18:645–8.5). Gentschev I., Dietrich G., Goebel W. The E. coli alpha-hemolysin secretion system and its use in vaccine development. Trends Microbiol. 2002. 10:39–45.6). Zhu C., Ruiz-Perez F., Yang Z., Mao Y., Hackethal VL., Greco KM., Choy W., Davis K., Butterton JR., Boedeker EC. Delivery of heterologous protein antigens via hemolysin or autotransporter systems by an attenuated ler mutant of rabbit enteropathogenic Escherichia coli. Vaccine. 2006. 24:3821–31.7). Wallace AJ., Stillman TJ., Atkins A., Jamieson SJ., Bullough PA., Green J., Artymiuk PJ. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell. 2000. 100:265–76.8). Wai SN., Lindmark B., Söderblom T., Takade A., Westermark M., Oscarsson J., Jass J., Richter-Dahlfors A., Mizunoe Y., Uhlin BE. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003. 115:25–35.
Article9). del Castillo FJ., Moreno F., del Castillo I. Secretion of the Escherichia coli K-12 SheA hemolysin is independent of its cytolytic activity. FEMS Microbiol Lett. 2001. 204:281–5.10). Galen JE., Zhao L., Chinchilla M., Wang JY., Pasetti MF., Green J., Levine MM. Adaptation of the endogenous Salmonella enterica serovar Typhi clyA-encoded hemolysin for antigen export enhances the immunogenicity of anthrax protective antigen domain 4 expressed by the attenuated live-vector vaccine strain CVD 908-htrA. Infect Immun. 2004. 72:7096–106.11). Spiga O., Bernini A., Ciutti A., Chiellini S., Menciassi N., Finetti F., Causarono V., Anselmi F., Prischi F., Niccolai N. Molecular modelling of S1 and S2 subunits of SARS coronavirus spike glycoprotein. Biochem Biophys Res Commun. 2003. 310:78–83.
Article12). Li W., Moore MJ., Vasilieva N., Sui J., Wong SK., Berne MA., Somasundaran M., Sullivan JL., Luzuriaga K., Greenough TC., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003. 426:450–4.
Article13). Wong SK., Li W., Moore MJ., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004. 279:3197–201.
Article14). He Y., Zhou Y., Liu S., Kou Z., Li W., Farzan M., Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004. 324:773–81.
Article15). Valle E., Guiney DG. Characterization of Salmonella-induced cell death in human macrophage-like THP-1 cells. Infect Immun. 2005. 73:2835–40.16). Kang HY., Srinivasan J., Curtiss R 3rd. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar typhimurium vaccine. Infect Immun. 2002. 70:1739–49.17). Song M., Kim HJ., Kim EY., Shin M., Lee HC., Hong Y., Rhee JH., Yoon H., Ryu S., Lim S., Choy HE. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J Biol Chem. 2004. 279:34183–90.18). Galan JE., Nakayama K., Curtiss R 3rd. Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene. 1990. 94:29–35.19). Nayak AR., Tinge SA., Tart RC., McDaniel LS., Briles DE., Curtiss R 3rd. A live recombinant avirulent oral Salmonella vaccine expressing pneumococcal surface protein A induces protective responses against Streptococcus pneumoniae. Infect Immun. 1998. 66:3744–51.20). Na HS., Kim HJ., Lee HC., Hong Y., Rhee JH., Choy HE. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine. 2006. 24:2027–34.21). Westermark M., Oscarsson J., Mizunoe Y., Urbonaviciene J., Uhlin BE. Silencing and activation of ClyA cytotoxin expression in Escherichia coli. J Bacteriol. 2000. 182:6347–57.22). Morales C., Lee MD., Hofacre C., Maurer JJ. Detection of a novel virulence gene and a Salmonella virulence homologue among Escherichia coli isolated from broiler chickens. Foodborne Pathog Dis. 2004. 1:160–5.23). Lai XH., Arencibia I., Johansson A., Wai SN., Oscarsson J., Kalfas S., Sundqvist KG., Mizunoe Y., Sjöstedt A., Uhlin BE. Cytocidal and apoptotic effects of the ClyA protein from Escherichia coli on primary and cultured monocytes and macrophages. Infect Immun. 2000. 68:4363–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Salmonella Typhi Osteomyelitis in a Non-sickle Cell Patient: Three Cases Report
- Detection od salmonella typhi by polymerase chain reaction
- A case of sphenoid sinusitis associated with salmonellosis by salmonella typhi group D
- Polymerase chain reaction for detection of H1-j strains of salmonella typhi
- A case of arthritis of hip joint caused by salmonella typhi