J Korean Surg Soc.
2009 Jul;77(1):7-14. 10.4174/jkss.2009.77.1.7.
Effects of Three Different Types of Anti-adhesive Agents in a Rat Abdominal Wall Defect Model
- Affiliations
-
- 1Department of Surgery, College of Medicine, Chung-Ang University, Seoul, Korea. Kimbg0526@paran.com
- 2Department of Pathology, College of Medicine, Chung-Ang University, Seoul, Korea.
- KMID: 1464937
- DOI: http://doi.org/10.4174/jkss.2009.77.1.7
Abstract
-
PURPOSE: Postsurgical adhesion formation is a significant clinical problem within every surgical specialty. Several adhesion barriers have been developed in the form of solution, membrane or film in an attempt to solve these problems. The purpose of the present study is to compare the efficacy of antiadhesive agents in the prevention of postsurgical adhesion formation in a standardized rat adhesion model.
METHODS
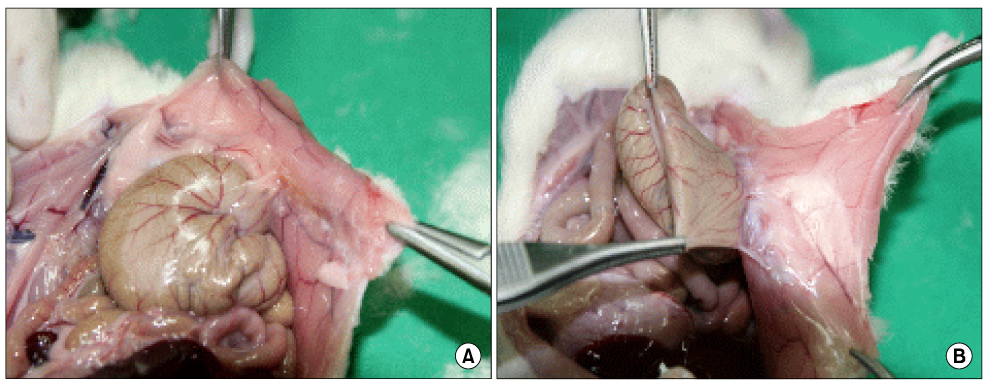
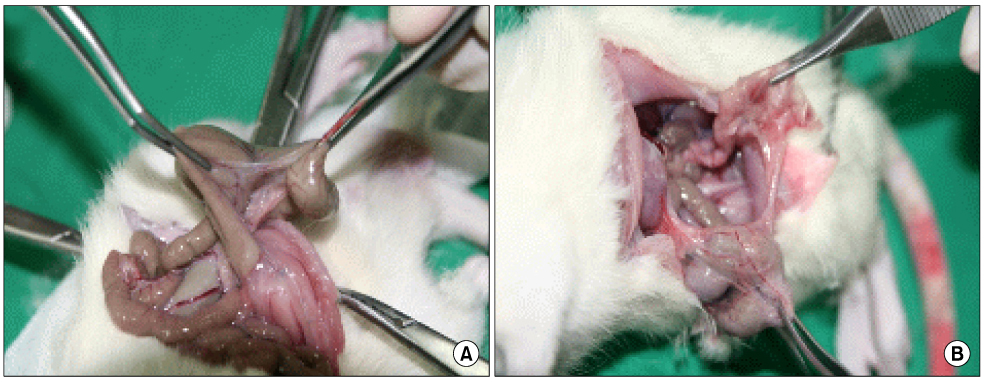
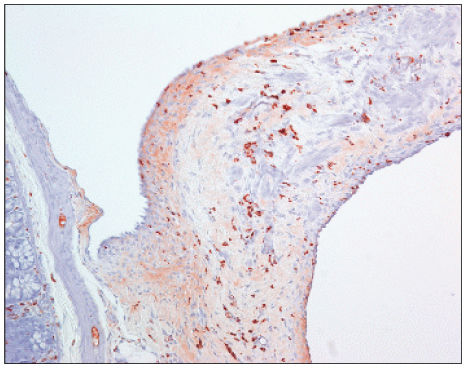
We examined forty Sprague-Dawley rats, which is a cecal abrasion with partial peritonectomy model. Three treatment groups (Group I: Film-type Surgiwrap(R), Group II: Solution-type Guardix-sol(R), Group III: Membrane-type Interceed(R)), each consists of 10 rats, and a control group of 10 rats were used by saline. Ten days after surgery, the rats were killed, and the levels of adhesion were graded. Immunohistochemical staining for microvessel density (CD34, MVD) and macrophage (ED1) were performed in adhesion tissue.
RESULTS
The peritoneum adhesion mean scores are as follows: control group: 2.2+/-0.78, Group I: 1.0+/-1.06, Group II: 0.9+/-0.99, Group III: 0.6+/-0.84. All treatment groups showed significantly less peritoneum adhesion (P=0.006), while there was no significant difference in each group. The intraperitoneal organs adhesion mean scores are as follows: control group: 2.8+/-0.91, Group I: 2.6+/-1.06, Group II: 1.4+/-0.84, Group III: 1.0+/-0.81. Group I had no significant difference about intraperitoneal organs adhesion with control group, but Group II and Group III showed less intraperitoneal organs adhesion. The mean numbers of microvessel density are as follows: control group: 42.5+/-4.83, Group I: 40.8+/-6.53, Group II: 30.9+/-6.15, Group III: 15.60+/-4.37, from which there was a significant difference between Group II and Group III with control group (P<0.001). The mean numbers of macrophage are as follows: control group: 223.3+/-33.12, Group I: 211.25+/-10.96, Group II: 171.60+/-23.96, Group III: 147.0+/-12.22, from which there was a significant difference between Group II and Group III with control group (P<0.001).
CONCLUSION
In our animal model, three different types of antiadhesive agents (Surgiwrap(R), Guardix-sol(R), Interceed(R)) were effective in adhesion prevention, but Surgiwrap(R) had less antiadhesive effect for intraperitoneal organs adhesion. Membrane-type Interceed(R) had a better effect for microvessel density (MVD) and macrophage than solution-type Guardix-sol(R).
MeSH Terms
Figure
Reference
-
1. Parker MC, Ellis H, Moran BJ, Thompson JN, Wilson MS, Menzies D, et al. Postoperative adhesions: ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Dis Colon Rectum. 2001. 44:822–829.2. Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998. 186:1–9.3. Hooker GD, Taylor BM, Driman DK. Prevention of adhesion formation with use of sodium hyaluronate-based bioresorbable membrane in a rat model of ventral hernia repair with polypropylene mesh--a randomized, controlled study. Surgery. 1999. 125:211–216.4. Knightly JJ, Agostino D, Cliffton EE. The effect of fibrinolysin and heparin on the formation of peritoneal adhesions. Surgery. 1962. 52:250–258.5. Epstein JC, Wilson MS, Wilkosz S, Ireland G, O'Dwyer ST, Herrick SE. Human peritoneal adhesions show evidence of tissue remodeling and markers of angiogenesis. Dis Colon Rectum. 2006. 49:1885–1892.6. Binnebösel M, Rosch R, Junge K, Lynen-Jansen P, Schumpelick V, Klinge U. Macrophage and T-lymphocyte infiltrates in human peritoneal adhesions indicate a chronic inflammatory disease. World J Surg. 2008. 32:296–304.7. Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, van Goor H. Recent clinical developments in pathophysiology, epidemiology, diagnosis and treatment of intra-abdominal adhesions. Scand J Gastroenterol Suppl. 2000. 232:52–59.8. Attard JA, MacLean AR. Adhesive small bowel obstruction: epidemiology, biology and prevention. Can J Surg. 2007. 50:291–300.9. Rout UK, Diamond MP. Role of plasminogen activators during healing after uterine serosal lesioning in the rat. Fertil Steril. 2003. 79:138–145.10. Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg. 1999. 165:1012–1019.11. Herrick SE, Mutsaers SE, Ozua P, Sulaiman H, Omer A, Boulos P, et al. Human peritoneal adhesions are highly cellular, innervated, and vascularized. J Pathol. 2000. 192:67–72.12. Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001. 7:556–566.13. Gutt CN, Oniu T, Schemmer P, Mehrabi A, Büchler MW. Fewer adhesions induced by laparoscopic surgery? Surg Endosc. 2004. 18:898–906.14. Bahadir I, Oncel M, Kement M, Sahip Y. Intra-abdominal use of taurolidine or heparin as alternative products to an anti-adhesive barrier (Seprafilm) in adhesion prevention: an experimental study on mice. Dis Colon Rectum. 2007. 50:2209–2214.15. Jackson EK. Intraperitoneal administration of adenosine inhibits formation of abdominal adhesions. Dis Colon Rectum. 2004. 47:1390–1396.16. Replogle RL, Johnson R, Gross RE. Prevention of postoperative intestinal adhesions with combined promethazine and dexamethasone therapy: experimental and clinical studies. Ann Surg. 1966. 163:580–588.17. Siegler AM, Kontopoulos V, Wang CF. Prevention of postoperative adhesions in rabbits with ibuprofen, a nonsteroidal anti-inflammatory agent. Fertil Steril. 1980. 34:46–49.18. de la Portilla F, Ynfante I, Bejarano D, Conde J, Fernandez A, Ortega JM, et al. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E: an experimental study in rats. Dis Colon Rectum. 2004. 47:2157–2161.19. Hellebrekers BW, Trimbos-Kemper TC, Trimbos JB, Emeis JJ, Kooistra T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000. 74:203–212.20. Montz FJ, Fowler JM, Wolff AJ, Lacey SM, Mohler M. The ability of recombinant tissue plasminogen activator to inhibit post-radical pelvic surgery adhesions in the dog model. Am J Obstet Gynecol. 1991. 165:1539–1542.21. Tander B, Bicakci U, Kilicoglu-Aydin B, Ariturk E, Rizalar R, Bernay F. Antiadhesive effects of mitomycin C and streptopeptidase A in rats with intraperitoneal adhesions. Pediatr Surg Int. 2007. 23:785–788.22. Shim HS, Lee YW, Lee YM, Oh YH, Kwon SW, Kim JH, et al. Evaluation of resorbable materials for preventing surgical adhesion on rat experiment. J Korean Surg Soc. 2002. 63:179–186.23. Ersoy E, Ozturk V, Yazgan A, Ozdogan M, Gundogdu H. Effect of polylactic acid film barrier on intra-abdominal adhesion formation. J Surg Res. 2008. 147:148–152.24. Lee YG, Chu B, Kim NH, Kim JH, Lee YW, Kim KI, et al. The efficacy and wafety of HA/CMC anti-adhesion barrier solution with varying viscosities. J Korean Surg Soc. 2008. 74:399–404.25. Gago LA, Saed G, Elhammady E, Diamond MP. Effect of oxidized regenerated cellulose (Interceed) on the expression of tissue plasminogen activator and plasminogen activator inhibitor-1 in human peritoneal fibroblasts and mesothelial cells. Fertil Steril. 2006. 86:1223–1227.26. Reddy S, Santanam N, Reddy PP, Rock JA, Murphy AA, Parthasarathy S. Interaction of Interceed oxidized regenerated cellulose with macrophages: a potential mechanism by which Interceed may prevent adhesions. Am J Obstet Gynecol. 1997. 177:1315–1320.27. Habara T, Nakatsuka M, Konishi H, Asagiri K, Noguchi S, Kudo T. The biological effects of antiadhesion agents on activated RAW264.7 macrophages. J Biomed Mater Res. 2002. 61:628–633.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Reconstruction of Abdominal Wall Defect using Tensor Fascia Lata Myocutaneous Flap: The Defect Caused by a Wide Resection of Squamous Cell Carcinoma of Bladder Invading Abdominal Wall
- A Case of Abdominal Wall Abscess
- Traumatic Abdominal Wall Hernia (TAWH): Repair by using a Prolen Mesh
- The effect of adhesive thickness on microtensile bond strength to the cavity wall
- Anti-adhesive Effect of Poloxamer/Alginate/CaCl2 Mixture in the Rat Model