Electrolyte Blood Press.
2009 Jun;7(1):9-13. 10.5049/EBP.2009.7.1.9.
Renal Handling of Ammonium and Acid Base Regulation
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea. hyekim@chungbuk.ac.kr
- KMID: 1464783
- DOI: http://doi.org/10.5049/EBP.2009.7.1.9
Abstract
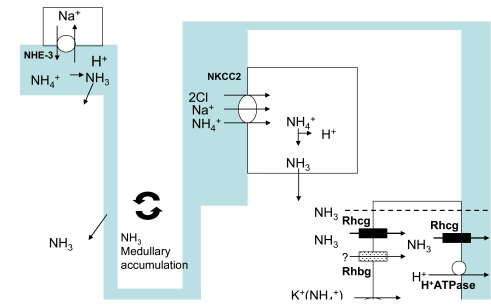
- Renal ammonium metabolism is the primary component of net acid excretion and thereby is critical for acid - base homeostasis. Briefly, ammonium is produced from glutamine in the proximal tubule in a series of biochemical reactions that result in equimolar bicarbonate. Ammonium is predominantly secreted into the luminal fluid via the apical Na++xchanger, NHE3. The thick ascending limb of the loop of Henle reabsorbs luminal ammonium, predominantly by transport of NH4+y the apical Na++Cl - cotransporter, BSC1/NKCC2. This process results in renal interstitial ammonium accumulation. Finally, the collecting duct secretes ammonium from the renal interstitium into the luminal fluid. Although in past ammonium was believed to move across epithelia entirely by passive diffusion, an increasing number of studies demonstrated that specific proteins contribute to renal ammonium transport. Recent studies have yielded important new insights into the mechanisms of renal ammonium transport. In this review, we will discuss renal handling of ammonium, with particular emphasis on the transporters involved in this process.
Keyword
MeSH Terms
Figure
Reference
-
1. Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol. 1987; 253:F595–F605. PMID: 3310662.
Article2. Halperin ML, Jungas RL. Metabolic production and renal disposal of hydrogen ions. Kidney Int. 1983; 24:709–713. PMID: 6674669.
Article3. DuBose TD Jr, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol. 1991; 1:1193–1203. PMID: 1932632.
Article4. Knepper MA. NH4+ transport in the kidney. Kidney Int Suppl. 1991; 40:S95–S102. PMID: 1890804.5. Good DW, Burg MB. Ammonia production by individual segments of the rat nephron. J Clin Invest. 1984; 73:602–610. PMID: 6323523.
Article6. May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986; 77:614–621. PMID: 3511100.
Article7. Nagami GT. Ammonia production and secretion by the proximal tubule. Am J Kidney Dis. 1989; 14:258–261. PMID: 2679054.
Article8. Simon EE, Merli C, Herndon J, Cragoe EJ Jr, Hamm LL. Effects of barium and 5-(N-ethyl-N-isopropyl)-amiloride on proximal tubule ammonia transport. Am J Physiol. 1992; 262:F36–F39. PMID: 1733295.
Article9. Hamm LL, Simon EE. Ammonia transport in the proximal tubule. Miner Electrolyte Metab. 1990; 16:283–290. PMID: 2178214.10. DuBose TD Jr, Good DW. Effects of chronic hyperkalemia on renal production and proximal tubule transport of ammonium in rats. Am J Physiol. 1991; 260:F680–F687. PMID: 2035655.
Article11. Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest. 1988; 81:159–164. PMID: 3121674.
Article12. Nagami GT. Ammonia production and secretion by S3 proximal tubule segments from acidotic mice: role of ANG II. Am J Physiol Renal Physiol. 2004; 287:F707–F712. PMID: 15345494.
Article13. Buerkert J, Martin D, Trigg D. Segmental analysis of the renal tubule in buffer production and net acid formation. Am J Physiol. 1983; 244:F442–F454. PMID: 6837741.
Article14. Packer RK, Desai SS, Hornbuckle K, Knepper MA. Role of countercurrent multiplication in renal ammonium handling: regulation of medullary ammonium accumulation. J Am Soc Nephrol. 1991; 2:77–83. PMID: 1912412.
Article15. Garvin JL, Burg MB, Knepper MA. Active NH4+ absorption by the thick ascending limb. Am J Physiol. 1988; 255:F57–F65. PMID: 3394813.16. Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature. 1989; 339:478–480. PMID: 2725680.
Article17. Good DW. Adaptation of HCO3- and NH4+ transport in rat MTAL: effects of chronic metabolic acidosis and Na+ intake. Am J Physiol. 1990; 258:F1345–F1353. PMID: 2337153.18. Attmane-Elakeb A, Mount DB, Sibella V, Vernimmen C, Hebert SC, Bichara M. Stimulation by in vivo and in vitro metabolic acidosis of expression of rBSC-1, the Na+-K+(NH4+)-2Cl- cotransporter of the rat medullary thick ascending limb. J Biol Chem. 1998; 273:33681–33691. PMID: 9837954.19. Wall SM. NH4+ augments net acid secretion by a ouabain-sensitive mechanism in isolated perfused inner medullary collecting ducts. Am J Physiol. 1996; 270:F432–F439. PMID: 8780245.20. Sajo IM, Goldstein MB, Sonnenberg H, Stinebaugh BJ, Wilson DR, Halperin ML. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1981; 20:353–358. PMID: 7300125.
Article21. Pitts RF. Renal excretion of acid. Fed Proc. 1948; 7:418–426. PMID: 18867436.22. Pitts RF. The role of ammonia production and excretion in regulation of acid-base balance. N Engl J Med. 1971; 284:32–38. PMID: 4922348.
Article23. Flessner MF, Wall SM, Knepper MA. Permeabilities of rat collecting duct segments to NH3 and NH4+. Am J Physiol. 1991; 260:F264–F272. PMID: 1996677.24. Wall SM. Ammonium transport and the role of the Na,K-ATPase. Miner Electrolyte Metab. 1996; 22:311–317. PMID: 8933502.25. Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol. 2007; 69:317–340. PMID: 17002591.
Article26. Wall SM, Koger LM. NH4+ transport mediated by Na(+)-K(+)-ATPase in rat inner medullary collecting duct. Am J Physiol. 1994; 267:F660–F670. PMID: 7943362.27. Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ. Contribution of the Na+-K+-2Cl- cotransporter NKCC1 to Cl- secretion in rat OMCD. Am J Physiol Renal Physiol. 2001; 280:F913–F921. PMID: 11292635.28. Ikebe M, Nonoguchi H, Nakayama Y, Tashima Y, Tomita K. Upregulation of the secretory-type Na(+)/K(+)/2Cl(-)-cotransporter in the kidney by metabolic acidosis and dehydration in rats. J Am Soc Nephrol. 2001; 12:423–430. PMID: 11181789.
Article29. Heitman J, Agre P. A new face of the Rhesus antigen. Nat Genet. 2000; 26:258–259. PMID: 11062455.
Article30. Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, et al. Expression of the ammonia transporter, Rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol. 2006; 17:2670–2679. PMID: 16928804.
Article31. Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, et al. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol. 2003; 14:545–554. PMID: 12595489.
Article32. Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Han KH, et al. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006; 290:F397–F408. PMID: 16144966.
Article33. Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol. 2003; 284:F323–F337. PMID: 12388412.34. Weiner ID, Verlander JW. Renal and hepatic expression of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein. Acta Physiol Scand. 2003; 179:331–338. PMID: 14656370.
Article35. Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, et al. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol. 2007; 293:F1238–F1247. PMID: 17652373.
Article36. Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, et al. Changes in subcellular distribution of the ammonia transporter, Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006; 290:F1443–F1452. PMID: 16434569.
Article37. Nakhoul NL, Dejong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4(+) transporter. Am J Physiol Renal Physiol. 2005; 288:F170–F181. PMID: 15353405.
Article38. Nakhoul NL, Schmidt E, Abdulnour-Nakhoul SM, Hamm LL. Electrogenic ammonium transport by renal Rhbg. Transfus Clin Biol. 2006; 13:147–153. PMID: 16580864.39. Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of ammonia transport by the kidney Rh glycoproteins RhBG and RhCG. Am J Physiol Renal Physiol. 2006; 290:F297–F305. PMID: 16131648.
Article40. Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, et al. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature. 2008; 456:339–343. PMID: 19020613.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renal Sodium Handling and Hypertension
- Altered Regulation of Renal Acid Base Transporters in Response to Ammonium Chloride Loading in Rats
- Renal Tubular Acidosis in Cadmium-Intoxicated Rats
- Effect of Mineralocorticoid on Serum Potassium Regulation and Urine Ammonium Excretion in Chronic Renal Patients
- Clinical Usefulness of Transtubular Potassium Gradient(TTKG) and Urine Ammonium in Differential Diagnosis of Hypokalemia