Anat Cell Biol.
2011 Jun;44(2):128-134. 10.5115/acb.2011.44.2.128.
ID4 mediates proliferation of astrocytes after excitotoxic damage in the mouse hippocampus
- Affiliations
-
- 1Department of Anatomy, School of Medicine, Chungnam National University, Daejeon, Korea. visnu528@cnu.ac.kr
- 2Department of Pediatrics, School of Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 1447422
- DOI: http://doi.org/10.5115/acb.2011.44.2.128
Abstract
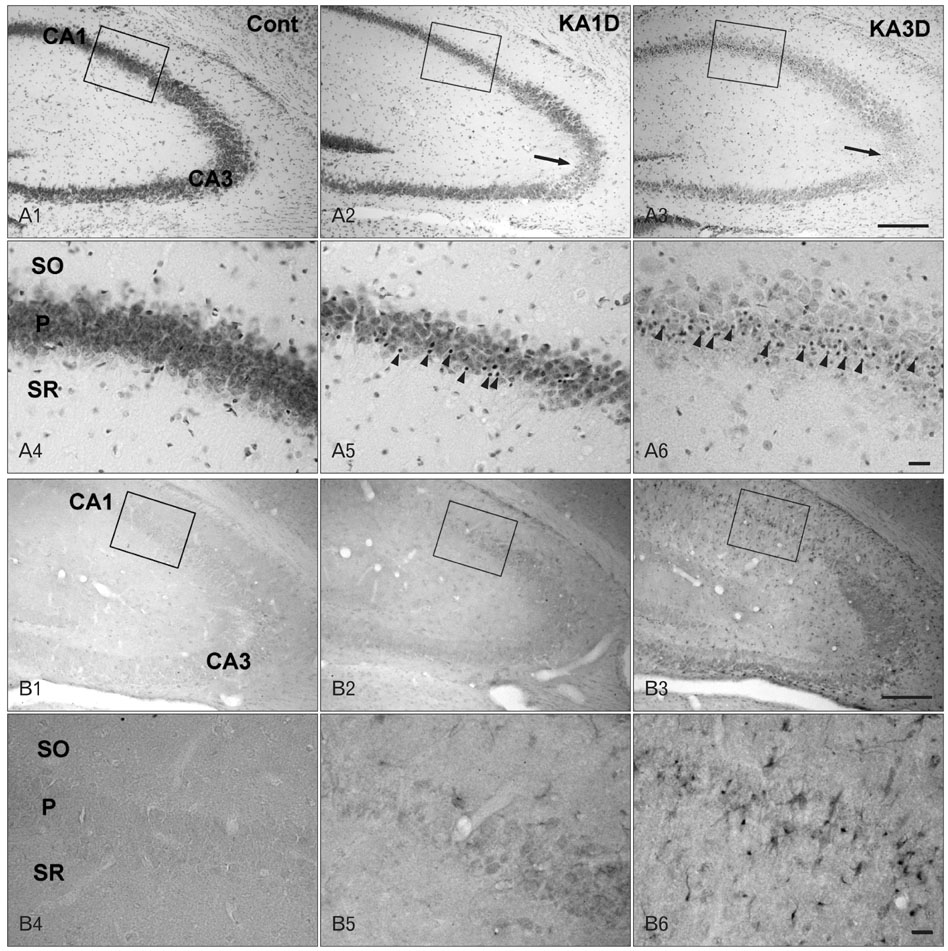
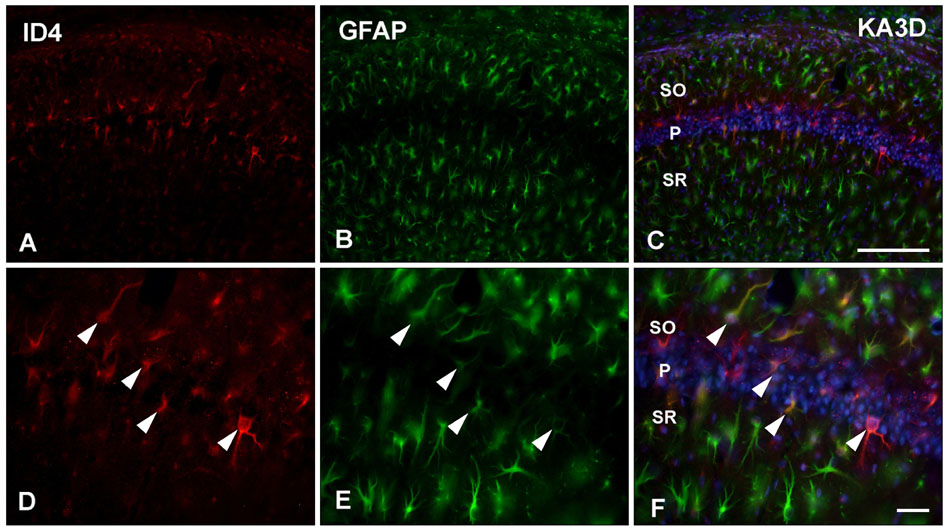
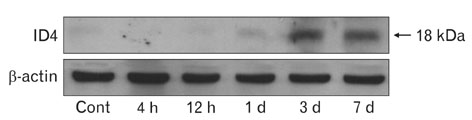
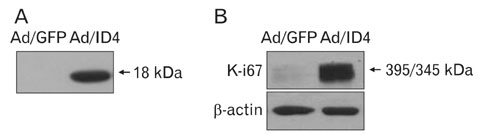
- Inhibitor of DNA binding (ID) proteins bind to and inhibit the function of basic helix-loop-helix transcription factors, including those that regulate proliferation and differentiation during development. However, little is known about the role of ID proteins in glial activation under neuropathological conditions. In this study, we evaluated the expression of ID4 following induction of excitotoxic lesions in mouse brain by kainic acid injection. The number of ID4-expressing astrocytes increased in the CA1 layer of the injured hippocampus until 3 days post-lesion. To analyze the effects of ID4 on cell proliferation, primary astrocytes were transduced with recombinant adenovirus expressing GFP-ID4. Overexpression of ID4 led to increased proliferation of astrocytes. These results suggest that ID4 plays a proliferative role in astrocyte activation after excitotoxin-induced hippocampal neuronal death.
Keyword
MeSH Terms
Figure
Reference
-
1. de Candia P, Benera R, Solit DB. A role for Id proteins in mammary gland physiology and tumorigenesis. Adv Cancer Res. 2004. 92:81–94.2. Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005. 5:603–614.3. Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000. 113(Pt 22):3897–3905.4. Andres-Barquin PJ, Hernandez MC, Israel MA. Id genes in nervous system development. Histol Histopathol. 2000. 15:603–618.5. Jen Y, Manova K, Benezra R. Each member of the Id gene family exhibits a unique expression pattern in mouse gastrulation and neurogenesis. Dev Dyn. 1997. 208:92–106.6. Riechmann V, Sablitzky F. Mutually exclusive expression of two dominant-negative helix-loop-helix (dnHLH) genes, Id4 and Id3, in the developing brain of the mouse suggests distinct regulatory roles of these dnHLH proteins during cellular proliferation and differentiation of the nervous system. Cell Growth Differ. 1995. 6:837–843.7. Andres-Barquin PJ, Hernandez MC, Israel MA. Id4 expression induces apoptosis in astrocytic cultures and is down-regulated by activation of the cAMP-dependent signal transduction pathway. Exp Cell Res. 1999. 247:347–355.8. van Crüchten I, Cinato E, Fox M, King ER, Newton JS, Riechmann V, Sablitzky F. Structure, chromosomal localisation and expression of the murine dominant negative helix-loop-helix Id4 gene. Biochim Biophys Acta. 1998. 1443:55–64.9. Kondo T, Raff M. The Id4 HLH protein and the timing of oligodendrocyte differentiation. EMBO J. 2000. 19:1998–2007.10. Liang Y, Bollen AW, Nicholas MK, Gupta N. Id4 and FABP7 are preferentially expressed in cells with astrocytic features in oligodendrogliomas and oligoastrocytomas. BMC Clin Pathol. 2005. 5:6.11. Jeon HM, Jin X, Lee JS, Oh SY, Sohn YW, Park HJ, Joo KM, Park WY, Nam DH, DePinho RA, Chin L, Kim H. Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling. Genes Dev. 2008. 22:2028–2033.12. Nadler JV, Perry BW, Gentry C, Cotman CW. Degeneration of hippocampal CA3 pyramidal cells induced by intraventricular kainic acid. J Comp Neurol. 1980. 192:333–359.13. Kim DW, Lee JH, Park SK, Yang WM, Jeon GS, Lee YH, Chung CK, Cho SS. Astrocytic expressions of phosphorylated Akt, GSK3beta and CREB following an excitotoxic lesion in the mouse hippocampus. Neurochem Res. 2007. 32:1460–1468.14. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986. 16:355–357.15. Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martínez A, Perez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor gamma activation. J Biol Chem. 2005. 280:21453–21462.16. Lee YS, Mollah ML, Sohn KC, Shi G, Kim DH, Kim KH, Cho MJ, Kim S, Lee YH, Kim CD, Lee JH. ID3 mediates X-ray-induced apoptosis of keratinocytes through the regulation of beta-catenin. J Dermatol Sci. 2010. 60:138–142.17. Lee YS, Sohn KC, Jang S, Lee Y, Hwang C, Kim KH, Cho MJ, Kim CD, Lee JH. Anti-apoptotic role of S100A8 in X-ray irradiated keratinocytes. J Dermatol Sci. 2008. 51:11–18.18. Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, Lee KE, Karin M, Lee SJ. Role of microglial IKKbeta in kainic acid-induced hippocampal neuronal cell death. Brain. 2008. 131(Pt 11):3019–3033.19. Bedford L, Walker R, Kondo T, van Crüchten I, King ER, Sablitzky F. Id4 is required for the correct timing of neural differentiation. Dev Biol. 2005. 280:386–395.20. Yun K, Mantani A, Garel S, Rubenstein J, Israel MA. Id4 regulates neural progenitor proliferation and differentiation in vivo. Development. 2004. 131:5441–5448.21. Murad JM, Place CS, Ran C, Hekmatyar SK, Watson NP, Kauppinen RA, Israel MA. Inhibitor of DNA binding 4 (ID4) regulation of adipocyte differentiation and adipose tissue formation in mice. J Biol Chem. 2010. 285:24164–24173.22. Du Y, Yip HK. The expression and roles of inhibitor of DNA binding helix-loop-helix proteins in the developing and adult mouse retina. Neuroscience. 2011. 175:367–379.23. Fontemaggi G, Dell'Orso S, Trisciuoglio D, Shay T, Melucci E, Fazi F, Terrenato I, Mottolese M, Muti P, Domany E, Del Bufalo D, Strano S, Blandino G. The execution of the transcriptional axis mutant p53, E2F1 and ID4 promotes tumor neo-angiogenesis. Nat Struct Mol Biol. 2009. 16:1086–1093.24. Kuzontkoski PM, Mulligan-Kehoe MJ, Harris BT, Israel MA. Inhibitor of DNA binding-4 promotes angiogenesis and growth of glioblastoma multiforme by elevating matrix GLA levels. Oncogene. 2010. 29:3793–3802.25. Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969. 137:433–457.26. Ninomiya M, Yamashita T, Araki N, Okano H, Sawamoto K. Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci Lett. 2006. 403:63–67.27. Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002. 110:429–441.28. Parent JM, von dem Bussche N, Lowenstein DH. Prolonged seizures recruit caudal subventricular zone glial progenitors into the injured hippocampus. Hippocampus. 2006. 16:321–328.29. Shetty AK. Entorhinal axons exhibit sprouting in CA1 subfield of the adult hippocampus in a rat model of temporal lobe epilepsy. Hippocampus. 2002. 12:534–542.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Pioglitazone on Excitotoxic Neuronal Damage in the Mouse Hippocampus
- Microglial Expression of Phospho-AMP-Activatd Protein Kinase (AMPK) Following an Excitotoxic Lesion in the Mouse Hippocampus
- Astrocytic Expression of CTMP Following an Excitotoxic Lesion in the Mouse Hippocampus
- Growth Differentiation Factor 15 Expression in Astrocytes After Excitotoxic Lesion in the Mouse Hippocampus
- Induction of alphaB-crystallin in the hippocampus of KA-treated mouse brain