Lab Anim Res.
2011 Dec;27(4):293-299. 10.5625/lar.2011.27.4.293.
Peroxiredoxin I regulates the component expression of gamma-secretase complex causing the Alzheimer's disease
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources and Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2School of Biosystems & Biomaterials Science and Engineering, Seoul National University, Seoul, Korea.
- KMID: 1444961
- DOI: http://doi.org/10.5625/lar.2011.27.4.293
Abstract
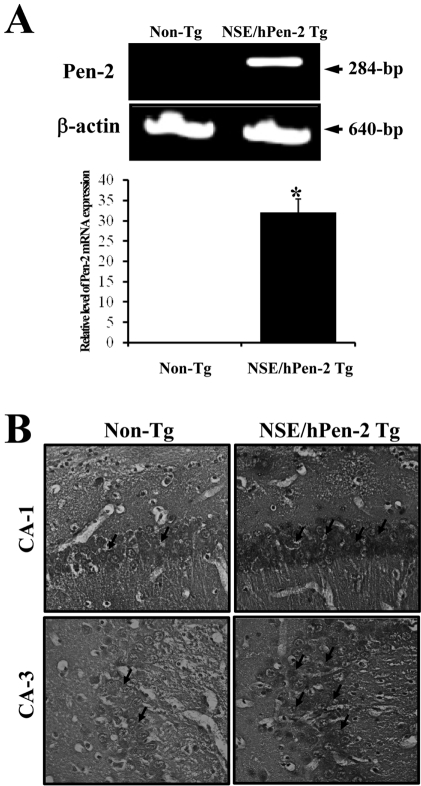
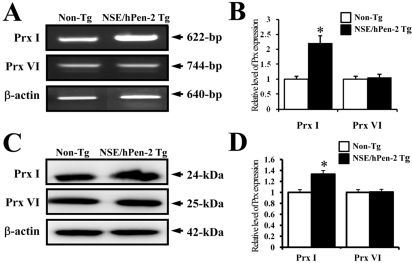
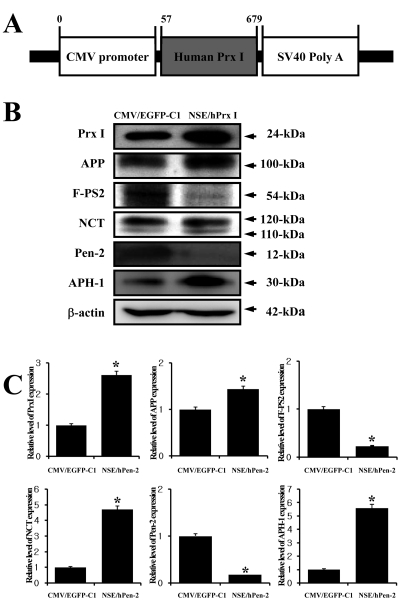
- Peroxiredoxin I (Prx I) is a member of the peroxiredoxins (Prxs) family, which are antioxidant enzymes that regulate various cellular process via intracellular oxidative signal pathways. In order to investigate the correlation between Prx I and the gamma-secretase complex, which causes Alzheimer's disease (AD), the expression level of Prx I was firstly evaluated in an animal model for AD. NSE/hPen-2 transgenic (Tg) mice, which were used as animal model in this study, showed a high level of Pen-2 expression and accumulation of Abeta-42 peptides in the hippocampus of brain. The expression level of Prx I was significantly higher on the mRNA and protein level in the brain of this model, while not change in Prx VI expression was observed. Furthermore, to verify the effect of Prx I on the gamma-secretase components in vitro, the expression level of these components was analyzed in the Prx I transfectants. Of the components of the gamma-secretase complex, the expression of PS-2 and Pen-2 was lower in the transfectants overexpressing Prx I compared to the vector transfectants. However, the expression of APP, NCT and APH-1 did not change in Prx I transfectants. Therefore, these results suggested that the expression of Prx I may be induced by the accumulation of Abeta-42 peptides and the overexpression of Prx I in neuroblastoma cells may regulate the expression of gamma-secretase components.
MeSH Terms
Figure
Reference
-
1. Hoidal JR. Reactive oxygen species and cell signaling. Am J Respir Cell Mol Biol. 2001; 25(6):661–663. PMID: 11726388.
Article2. Luo Y, Pang H, Li S, Cao H, Peng Z, Fan C, Li S. Production and radioimmunoimaging of novel fully human phage display recombinant antibodies and growth inhibition of lung adenocarcinoma cell line overexpressing Prx I. Cancer Biol Ther. 2009; 8(14):1369–1377. PMID: 19556853.
Article3. Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999; 300:219–226. PMID: 9919524.
Article4. Seo MS, Kang SW, Kim K, Baines IC, Lee TH, Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem. 2000; 275(27):20346–20354. PMID: 10751410.
Article5. Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J Biol Chem. 1998; 273(11):6297–6302. PMID: 9497357.
Article6. Okado-Matsumoto A, Matsumoto A, Fujii J, Taniguchi N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J Biochem. 2000; 127(3):493–501. PMID: 10731722.
Article7. Krapfenbauer K, Engidawork E, Cairns N, Fountoulakis M, Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003; 967(1-2):152–160. PMID: 12650976.
Article8. Cumming RC, Dargusch R, Fischer WH, Schubert D. Increase in expression levels and resistance to sulfhydryl oxidation of peroxiredoxin isoforms in amyloid beta-resistant nerve cells. J Biol Chem. 2007; 282(42):30523–30534. PMID: 17761673.9. Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature. 2000; 407(6800):48–54. PMID: 10993067.10. Li YM. Gamma-secretase: a catalyst of Alzheimer disease and signal transduction. Mol Interv. 2001; 1(4):198–207. PMID: 14993342.11. Nam SH, Seo SJ, Goo JS, Kim JE, Choi SI, Lee HR, Hwang IS, Jee SW, Lee SH, Bae CJ, Park JY, Kim HS, Shim SB, Hwang DY. Pen-2 overexpression induces Aβ-42 production, memory defect, motor activity enhancement and feeding behavior dysfunction in NSE/Pen-2 transgenic mice. Int J Mol Med. 2011; 28:961–971. PMID: 21822534.
Article12. Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song CW, Paik SG, Cho JS, Kim YK. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. 2002; 16:805–813. PMID: 12039862.13. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010; 1327:118–124. PMID: 20193666.
Article14. Kim HJ, Chae HZ, Kim YJ, Kim YH, Hwangs TS, Park EM, Park YM. Preferential elevation of Prx I and Trx expression in lung cancer cells following hypoxia and in human lung cancer tissues. Cell Biol Toxicol. 2003; 19(5):285–298. PMID: 14703116.
Article15. Chang JW, Lee SH, Jeong JY, Chae HZ, Kim YC, Park ZY, Yoo YJ. Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small cell lung cancer. FEBS Lett. 2005; 579(13):2873–2877. PMID: 15876430.
Article16. Nonn L, Berggren M, Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003; 1(9):682–689. PMID: 12861054.17. Oda A, Tamaoka A, Araki W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J Neurosci Res. 2010; 88(5):1137–1145. PMID: 19885829.
Article18. Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, Fang F, Chen JX, Yan SD, Gunn-Moore FJ. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mol Cell Neurosci. 2007; 35(2):377–382. PMID: 17490890.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overexpression of N141I PS2 increases γ-secretase activity through up-regulation of Presenilin and Pen-2 in brain mitochondria of NSE/hPS2m transgenic mice
- Altered expression of gamma-secretase components in animal model of major depressive disorder induced by reserpine administration
- Optogenetic neuromodulation with gamma oscillation as a new strategy for Alzheimer disease: a narrative review
- Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and gamma-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease
- Effect of Ischemic Neuronal Insults on Amyloid Precursor Protein Processing