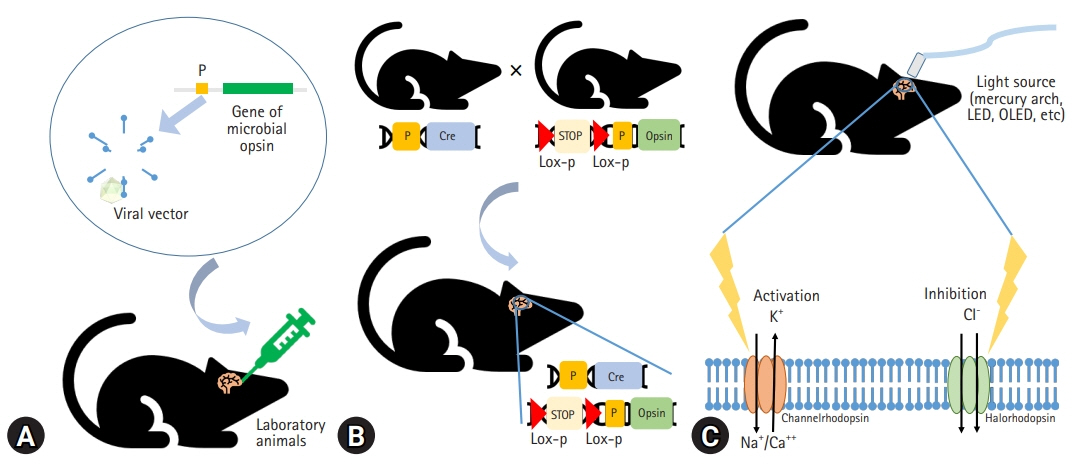
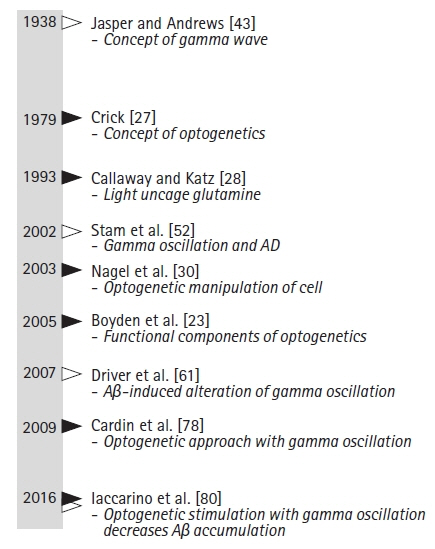
J Yeungnam Med Sci.
2022 Oct;39(4):269-277. 10.12701/jyms.2021.01683.
Optogenetic neuromodulation with gamma oscillation as a new strategy for Alzheimer disease: a narrative review
- Affiliations
-
- 1Medical Course, Jeju National University School of Medicine, Jeju, Korea
- 2Department of Anatomy, Jeju National University College of Medicine, Jeju, Korea
- KMID: 2534653
- DOI: http://doi.org/10.12701/jyms.2021.01683
Abstract
- The amyloid hypothesis has been considered a major explanation of the pathogenesis of Alzheimer disease. However, failure of phase III clinical trials with anti-amyloid-beta monoclonal antibodies reveals the need for other therapeutic approaches to treat Alzheimer disease. Compared to its relatively short history, optogenetics has developed considerably. The expression of microbial opsins in cells using genetic engineering allows specific control of cell signals or molecules. The application of optogenetics to Alzheimer disease research or clinical approaches is increasing. When applied with gamma entrainment, optogenetic neuromodulation can improve Alzheimer disease symptoms. Although safety problems exist with optogenetics such as the use of viral vectors, this technique has great potential for use in Alzheimer disease. In this paper, we review the historical applications of optogenetic neuromodulation with gamma entrainment to investigate the mechanisms involved in Alzheimer disease and potential therapeutic strategies.
Figure
Reference
-
References
1. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021; 17:327–406.2. Lee JS, Kang MJ, Nam HJ, Kim YJ, Lee OJ, Kim KO. Korean dementia observatory 2019 (Report No. NIDR-1902-0028) [Internet]. Seoul: Central Dementia Center Service;2020. [cited 2021 Dec 09]. https://www.nid.or.kr/info/dataroom_view.aspx?bid=209.3. Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009; 338:b158.
Article4. Nimmrich V, Draguhn A, Axmacher N. Neuronal network oscillations in neurodegenerative diseases. Neuromolecular Med. 2015; 17:270–84.
Article5. Canter RG, Penney J, Tsai LH. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 2016; 539:187–96.
Article6. Strüber D, Herrmann CS. Modulation of gamma oscillations as a possible therapeutic tool for neuropsychiatric diseases: a review and perspective. Int J Psychophysiol. 2020; 152:15–25.
Article7. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011; 377:1019–31.
Article8. Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984; 122:1131–5.
Article9. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016; 8:595–608.
Article10. Cui X, Zhang F, Zhang H, Huang X, Wang K, Huang T, et al. Neuroprotective effect of optogenetics varies with distance from channelrhodopsin-2 expression in an amyloid-β-injected mouse model of Alzheimer’s disease. Front Neurosci. 2020; 14:583628.
Article11. Gao Y, Tan L, Yu JT, Tan L. Tau in Alzheimer’s disease: mechanisms and therapeutic strategies. Curr Alzheimer Res. 2018; 15:283–300.
Article12. Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991; 6:487–98.
Article13. Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992; 256:184–5.
Article14. Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001; 414:643–8.
Article15. Hooper NM. Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein. Biochem Soc Trans. 2005; 33(Pt 2):335–8.
Article16. Ohnishi S, Takano K. Amyloid fibrils from the viewpoint of protein folding. Cell Mol Life Sci. 2004; 61:511–24.
Article17. Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004; 62:1984–9.
Article18. Citron M. Strategies for disease modification in Alzheimer’s disease. Nat Rev Neurosci. 2004; 5:677–85.
Article19. Oxford AE, Stewart ES, Rohn TT. Clinical trials in Alzheimer’s disease: a hurdle in the path of remedy. Int J Alzheimers Dis. 2020; 2020:5380346.
Article20. Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993; 90:567–71.
Article21. Ekinci FJ, Linsley MD, Shea TB. Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res Mol Brain Res. 2000; 76:389–95.22. Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004; 1742:81–7.23. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005; 8:1263–8.
Article24. Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006; 26:10380–6.
Article25. Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012; 13:251–66.
Article26. Byun J. Optogenetics: a new frontier for cell physiology study. J Life Sci. 2015; 25:953–9.
Article27. Crick F. The impact of molecular biology on neuroscience. Philos Trans R Soc Lond B Biol Sci. 1999; 354:2021–5.
Article28. Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci U S A. 1993; 90:7661–5.
Article29. Zemelman BV, Lee GA, Ng M, Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002; 33:15–22.
Article30. Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003; 100:13940–5.
Article31. Pama EA, Colzato LS, Hommel B. Optogenetics as a neuromodulation tool in cognitive neuroscience. Front Psychol. 2013; 4:610.
Article32. Beck S, Yu-Strzelczyk J, Pauls D, Constantin OM, Gee CE, Ehmann N, et al. Synthetic light-activated ion channels for optogenetic activation and inhibition. Front Neurosci. 2018; 12:643.
Article33. Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008; 36:129–39.
Article34. Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010; 330:1677–81.
Article35. Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010; 5:439–56.
Article36. Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011; 34:389–412.
Article37. Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007; 54:205–18.
Article38. Schindler SE, McCall JG, Yan P, Hyrc KL, Li M, Tucker CL, et al. Photo-activatable Cre recombinase regulates gene expression in vivo. Sci Rep. 2015; 5:13627.
Article39. Kawano F, Okazaki R, Yazawa M, Sato M. A photoactivatable Cre-loxP recombination system for optogenetic genome engineering. Nat Chem Biol. 2016; 12:1059–64.
Article40. Matarèse BF, Feyen PL, de Mello JC, Benfenati F. Sub-millisecond control of neuronal firing by organic light-emitting diodes. Front Bioeng Biotechnol. 2019; 7:278.
Article41. Mahmoudi P, Veladi H, Pakdel FG. Optogenetics, tools and applications in neurobiology. J Med Signals Sens. 2017; 7:71–9.
Article42. Malyshev A, Goz R, LoTurco JJ, Volgushev M. Advantages and limitations of the use of optogenetic approach in studying fast-scale spike encoding. PLoS One. 2015; 10:e0122286.
Article43. Jasper HH, Andrews HL. Electro-encephalography: III. Normal differentiation of occipital and precentral regions in man. Arch Neurol Psychiatr. 1938; 39:96–115.44. McDermott B, Porter E, Hughes D, McGinley B, Lang M, O’Halloran M, et al. Gamma band neural stimulation in humans and the promise of a new modality to prevent and treat Alzheimer’s disease. J Alzheimers Dis. 2018; 65:363–92.
Article45. Esmaeilpour Z, Kronberg G, Reato D, Parra LC, Bikson M. Temporal interference stimulation targets deep brain regions by modulating neural oscillations. Brain Stimul. 2021; 14:55–65.
Article46. Yuan Y, Yan J, Ma Z, Li X. Effect of noninvasive focused ultrasound stimulation on gamma oscillations in rat hippocampus. Neuroreport. 2016; 27:508–15.
Article47. Etter G, van der Veldt S, Manseau F, Zarrinkoub I, Trillaud-Doppia E, Williams S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat Commun. 2019; 10:5322.
Article48. Herrmann CS, Fründ I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev. 2010; 34:981–92.
Article49. Sohal VS. How close are we to understanding what (if anything) γ oscillations do in cortical circuits? J Neurosci. 2016; 36:10489–95.
Article50. Adaikkan C, Tsai LH. Gamma entrainment: impact on neurocircuits, glia, and therapeutic opportunities. Trends Neurosci. 2020; 43:24–41.
Article51. Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007; 8:45–56.
Article52. Stam CJ, van Cappellen van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, Scheltens P, et al. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002; 19:562–74.
Article53. Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand. 2003; 108:90–6.
Article54. Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, et al. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2005; 26:165–71.
Article55. van Deursen JA, Vuurman EF, Verhey FR, van Kranen-Mastenbroek VH, Riedel WJ. Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm (Vienna). 2008; 115:1301–11.
Article56. Başar E, Emek-Savaş DD, Güntekin B, Yener GG. Delay of cognitive gamma responses in Alzheimer’s disease. Neuroimage Clin. 2016; 11:106–15.
Article57. Mably AJ, Colgin LL. Gamma oscillations in cognitive disorders. Curr Opin Neurobiol. 2018; 52:182–7.
Article58. Klein AS, Donoso JR, Kempter R, Schmitz D, Beed P. Early cortical changes in Gamma oscillations in Alzheimer’s disease. Front Syst Neurosci. 2016; 10:83.
Article59. da Silva VF, Ribeiro AP, Dos Santos VA, Nardi AE, King AL, Calomeni MR. Stimulation by light and sound: therapeutics effects in humans: systematic review. Clin Pract Epidemiol Ment Health. 2015; 11:150–4.
Article60. Calomeni MR, Furtado da Silva V, Velasques BB, Feijó OG, Bittencourt JM, Ribeiro de Souza E Silva AP. Modulatory effect of association of brain stimulation by light and binaural beats in specific brain waves. Clin Pract Epidemiol Ment Health. 2017; 13:134–44.
Article61. Driver JE, Racca C, Cunningham MO, Towers SK, Davies CH, Whittington MA, et al. Impairment of hippocampal gamma-frequency oscillations in vitro in mice overexpressing human amyloid precursor protein (APP). Eur J Neurosci. 2007; 26:1280–8.62. Peña-Ortega F. Amyloid beta-protein and neural network dysfunction. J Neurodegener Dis. 2013; 2013:657470.
Article63. Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011; 71:9–34.
Article64. Kellar KJ, Whitehouse PJ, Martino-Barrows AM, Marcus K, Price DL. Muscarinic and nicotinic cholinergic binding sites in Alzheimer’s disease cerebral cortex. Brain Res. 1987; 436:62–8.
Article65. Marutle A, Warpman U, Bogdanovic N, Nordberg A. Regional distribution of subtypes of nicotinic receptors in human brain and effect of aging studied by (+/-)-[3H]epibatidine. Brain Res. 1998; 801:143–9.66. Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999; 289:1545–52.67. Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M, et al. Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur J Pharmacol. 2000; 393:215–22.
Article68. Bell KA, Shim H, Chen CK, McQuiston AR. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain α4 and β2 subunits. Neuropharmacology. 2011; 61:1379–88.
Article69. Yang X, Yao C, Tian T, Li X, Yan H, Wu J, et al. A novel mechanism of memory loss in Alzheimer’s disease mice via the degeneration of entorhinal-CA1 synapses. Mol Psychiatry. 2018; 23:199–210.
Article70. Jarzebowski P, Tang CS, Paulsen O, Hay YA. Impaired spatial learning and suppression of sharp wave ripples by cholinergic activation at the goal location. Elife. 2021; 10:e65998.
Article71. Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS One. 2012; 7:e40555.
Article72. Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016; 531:508–12.
Article73. Perusini JN, Cajigas SA, Cohensedgh O, Lim SC, Pavlova IP, Donaldson ZR, et al. Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer’s disease mice. Hippocampus. 2017; 27:1110–22.
Article74. Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, et al. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006; 142:941–52.
Article75. Dall’Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp Neurol. 2007; 203:241–5.76. Cunha GM, Canas PM, Melo CS, Hockemeyer J, Müller CE, Oliveira CR, et al. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008; 210:776–81.77. Li P, Rial D, Canas PM, Yoo JH, Li W, Zhou X, et al. Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry. 2015; 20:1339–49.
Article78. Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009; 459:663–7.
Article79. Thomson H. How flashing lights and pink noise might banish Alzheimer’s, improve memory and more. Nature. 2018; 555:20–2.
Article80. Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016; 540:230–5.
Article81. Zhang Z, Jing Y, Ma Y, Duan D, Li B, Hölscher C, et al. Driving GABAergic neurons optogenetically improves learning, reduces amyloid load and enhances autophagy in a mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2020; 525:928–35.
Article82. Park K, Lee J, Jang HJ, Richards BA, Kohl MM, Kwag J. Optogenetic activation of parvalbumin and somatostatin interneurons selectively restores theta-nested gamma oscillations and oscillation-induced spike timing-dependent long-term potentiation impaired by amyloid β oligomers. BMC Biol. 2020; 18:7.83. Chung H, Park K, Jang HJ, Kohl MM, Kwag J. Dissociation of somatostatin and parvalbumin interneurons circuit dysfunctions underlying hippocampal theta and gamma oscillations impaired by amyloid β oligomers in vivo. Brain Struct Funct. 2020; 225:935–54.
Article84. Wilson CA, Fouda S, Sakata S. Effects of optogenetic stimulation of basal forebrain parvalbumin neurons on Alzheimer’s disease pathology. Sci Rep. 2020; 10:15456.
Article85. Kastanenka KV, Calvo-Rodriguez M, Hou SS, Zhou H, Takeda S, Arbel-Ornath M, et al. Frequency-dependent exacerbation of Alzheimer’s disease neuropathophysiology. Sci Rep. 2019; 9:8964.
Article86. Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. 2020; 27:18.
Article87. Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999; 286:2244–5.
Article88. Kim GU, Kim HI, Chung E. Towards human clinical application of emerging optogenetics technology. Biomed Eng Lett. 2011; 1:207–12.
Article89. Bostancıklıoğlu M. An update on memory formation and retrieval: an engram-centric approach. Alzheimers Dement. 2020; 16:926–37.
Article90. Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, et al. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat Neurosci. 2013; 16:613–21.
Article91. Yamamoto K, Tanei ZI, Hashimoto T, Wakabayashi T, Okuno H, Naka Y, et al. Chronic optogenetic activation augments aβ pathology in a mouse model of Alzheimer disease. Cell Rep. 2015; 11:859–65.
Article92. Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016; 19:1085–92.
Article93. Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009; 324:354–9.
Article94. Zalocusky KA, Fenno LE, Deisseroth K. Current challenges in optogenetics. In : Hegemann P, Sigrist S, editors. Optogenetics. Berlin: De Gruyter;2013. p. 23–34.95. Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010; 466:622–6.
Article96. Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009; 324:1080–4.
Article97. Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS. Emerging therapies in Parkinson disease: repurposed drugs and new approaches. Nat Rev Neurol. 2019; 15:204–23.
Article98. Berlinguer-Palmini R, Narducci R, Merhan K, Dilaghi A, Moroni F, Masi A, et al. Arrays of microLEDs and astrocytes: biological amplifiers to optogenetically modulate neuronal networks reducing light requirement. PLoS One. 2014; 9:e108689.
Article99. Iwai Y, Honda S, Ozeki H, Hashimoto M, Hirase H. A simple head-mountable LED device for chronic stimulation of optogenetic molecules in freely moving mice. Neurosci Res. 2011; 70:124–7.
Article100. Zhang Y, Castro DC, Han Y, Wu Y, Guo H, Weng Z, et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc Natl Acad Sci U S A. 2019; 116:21427–37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impairement of Gamma Oscillation in Patients with Schizophrenia
- Cardiovascular Complications of Acetylcholinesterase Inhibitors in Patients with Alzheimer’s Disease: A Narrative Review
- Correlation between Alteration of Sharp-wave Ripple Coupled Cortical Oscillation and Long-term Memory Deficit in Alzheimer Disease Model Mice
- Memorials of Alois Alzheimer (June 14, 1864~December 19, 1915) and Historical Background of Alzheimer's Disease
- Pulse-train Stimulation of Primary Somatosensory Cortex Blocks Pain Perception in Tail Clip Test