Lab Anim Res.
2011 Dec;27(4):265-273. 10.5625/lar.2011.27.4.265.
Diverse animal models to examine potential role(s) and mechanism of endocrine disrupting chemicals on the tumor progression and prevention: Do they have tumorigenic or anti-tumorigenic property?
- Affiliations
-
- 1Laboratory of Veterinary Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. kchoi@cbu.ac.kr
- KMID: 1444958
- DOI: http://doi.org/10.5625/lar.2011.27.4.265
Abstract
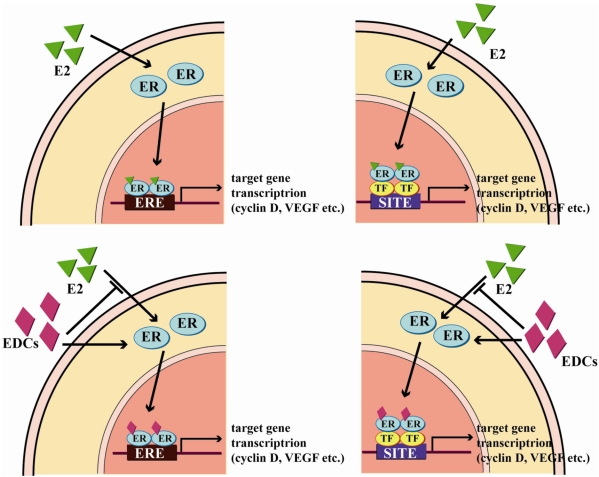
- Acting as hormone mimics or antagonists in the interaction with hormone receptors, endocrine disrupting chemicals (EDCs) have the potentials of disturbing the endocrine system in sex steroid hormone-controlled organs and tissues. These effects may lead to the disruption of major regulatory mechanisms, the onset of developmental disorders, and carcinogenesis. Especially, among diverse EDCs, xenoestrogens such as bisphenol A, dioxins, and di(2-ethylhexyl)phthalate, have been shown to activate estrogen receptors (ERs) and to modulate cellular functions induced by ERs. Furthermore, they appear to be closely related with carcinogenicity in estrogen-dependant cancers, including breast, ovary, and prostate cancers. In in vivo animal models, prenatal exposure to xenoestrogens changed the development of the mouse reproductive organs and increased the susceptibility to further carcinogenic exposure and tumor occurence in adults. Unlike EDCs, which are chemically synthesized, several phytoestrogens such as genistein and resveratrol showed chemopreventive effects on specific cancers by contending with ER binding and regulating normal ER action in target tissues of mice. These results support the notion that a diet containing high levels of phytoestrogens can have protective effects on estrogen-related diseases. In spite of the diverse evidences of EDCs and phytoestrogens on causation and prevention of estrogen-dependant cancers provided in this article, there are still disputable questions about the dose-response effect of EDCs or chemopreventive potentials of phytoestrogens. As a wide range of EDCs including phytoestrogens have been remarkably increasing in the environment with the rapid growth in our industrial society and more closely affecting human and wildlife, the potential risks of EDCs in endocrine disruption and carcinogenesis are important issues and needed to be verified in detail.
MeSH Terms
-
Adult
Animals
Benzhydryl Compounds
Breast
Diet
Dioxins
Endocrine Disruptors
Endocrine System
Estrogens
Female
Genistein
Humans
Mice
Models, Animal
Ovary
Phenols
Phytoestrogens
Prostatic Neoplasms
Receptors, Estrogen
Stilbenes
Benzhydryl Compounds
Dioxins
Endocrine Disruptors
Estrogens
Genistein
Phenols
Phytoestrogens
Receptors, Estrogen
Stilbenes
Figure
Reference
-
1. Murono EP, Derk RC, de Leon JH. Differential effects of octylphenol, 17β-estradiol, endosulfan, or bisphenol A on the steroidogenic competence of cultured adult rat Leydig cells. Reprod Toxicol. 2001; 15(5):551–560. PMID: 11780963.
Article2. Bredhult C, Backlin BM, Olovsson M. Effects of some endocrine disruptors on the proliferation and viability of human endometrial endothelial cells in vitro. Reprod Toxicol. 2007; 23(4):550–559. PMID: 17493787.3. Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011; 24(1):6–19. PMID: 21053929.
Article4. Takamiya M, Lambard S, Huhtaniemi IT. Effect of bisphenol A on human chorionic gonadotrophin-stimulated gene expression of cultured mouse Leydig tumour cells. Reprod Toxicol. 2007; 24(2):265–275. PMID: 17706920.
Article5. Harrison PT. Endocrine disrupters and human health. BMJ. 2001; 323(7325):1317–1318. PMID: 11739203.
Article6. Hwang KA, Park SH, Yi BR, Choi KC. Gene alterations of ovarian cancer cells expressing estrogen receptors by estrogen and bisphenol a using microarray analysis. Lab Anim Res. 2011; 27(2):99–107. PMID: 21826169.
Article7. Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol. 2010; 6(7):363–370. PMID: 20498677.
Article8. Barry JM, John M. Barry: distinguished scholar at the Center for Bioenvironmental Research, Tulane and Xavier Universities [Interview by Madeline Drexler]. Biosecur Bioterror. 2009; 7(2):127–133. PMID: 19485709.9. Shao ZM, Shen ZZ, Fontana JA, Barsky SH. Genistein's "ER-dependent and independent" actions are mediated through ER pathways in ER-positive breast carcinoma cell lines. Anticancer Res. 2000; 20(4):2409–2416. PMID: 10953303.10. Cotroneo MS, Wang J, Fritz WA, Eltoum IE, Lamartiniere CA. Genistein action in the prepubertal mammary gland in a chemoprevention model. Carcinogenesis. 2002; 23(9):1467–1474. PMID: 12189189.
Article11. Chen XW, Garner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem Biophys Res Commun. 2002; 295(2):417–422. PMID: 12150965.
Article12. Jenkins S, Betancourt AM, Wang J, Lamartiniere CA. Endocrine-active chemicals in mammary cancer causation and prevention. J Steroid Biochem Mol Biol. 2011; in press.
Article13. Ma L. Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol Metab. 2009; 20(7):357–363. PMID: 19709900.
Article14. Watanabe J, Kamata Y, Seo N, Okayasu I, Kuramoto H. Stimulatory effect of estrogen on the growth of endometrial cancer cells is regulated by cell-cycle regulators. J Steroid Biochem Mol Biol. 2007; 107(3-5):163–171. PMID: 17681750.
Article15. Craig ZR, Wang W, Flaws JA. Endocrine disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011; 142(5):633–646. PMID: 21862696.16. Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol. 2000; 15(4):1261–1270. PMID: 11005250.17. Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000; 74(5):311–317. PMID: 11162939.
Article18. Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S. Ligand-, cell-, and estrogen receptor subtype (α/β)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000; 275(8):5379–5387. PMID: 10681512.
Article19. Glidewell-Kenney C, Weiss J, Lee EJ, Pillai S, Ishikawa T, Ariazi EA, Jameson JL. ERE-independent ERα target genes differentially expressed in human breast tumors. Mol Cell Endocrinol. 2005; 245(1-2):53–59. PMID: 16298037.
Article20. Zhou Y, Yau C, Gray JW, Chew K, Dairkee SH, Moore DH, Eppenberger U, Eppenberger-Castori S, Benz CC. Enhanced NF-κB and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007; 7:59. PMID: 17407600.
Article21. Imaida K, Shirai T. Endocrine disrupting chemicals and carcinogenesis - breast, testis and prostate cancer. Nihon Rinsho. 2000; 58(12):2527–2532. PMID: 11187749.22. Hutchinson TH. Reproductive and developmental effects of endocrine disrupters in invertebrates: in vitro and in vivo approaches. Toxicol Lett. 2002; 131(1-2):75–81. PMID: 11988360.23. Swan SH. Intrauterine exposure to diethylstilbestrol: long-term effects in humans. APMIS. 2000; 108(12):793–804. PMID: 11252812.
Article24. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009; 30(4):293–342. PMID: 19502515.
Article25. Iwata M, Eshima Y, Kagechika H, Miyaura H. The endocrine disruptors nonylphenol and octylphenol exert direct effects on T cells to suppress Th1 development and enhance Th2 development. Immunol Lett. 2004; 94(1-2):135–139. PMID: 15234545.
Article26. Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002; 16(10):2188–2201. PMID: 12351685.27. Cheung E, Acevedo ML, Cole PA, Kraus WL. Altered pharmacology and distinct coactivator usage for estrogen receptor-dependent transcription through activating protein-1. Proc Natl Acad Sci USA. 2005; 102(3):559–564. PMID: 15642950.
Article28. Renner R. European bans on surfactant trigger transatlantic debate. Environ Sci Technol. 1997; 31(7):316A–320A.
Article29. Barrett JR. Estrogens from the outside in: alkylphenols, BPA disrupt ERK signaling in vitro. Environ Health Perspect. 2011; 119(1):A34. PMID: 21196144.
Article30. Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011; 119(6):878–885. PMID: 21233055.
Article31. Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERRγ. J Biochem. 2007; 142(4):517–524. PMID: 17761695.
Article32. Lamartiniere CA, Jenkins S, Betancourt AM, Wang J, Russo J. Exposure to the endocrine disruptor bisphenol A alters susceptibility for mammary cancer. Horm Mol Biol Clin Investig. 2011; 5(2):45–52.
Article33. Riu A, le Maire A, Grimaldi M, Audebert M, Hillenweck A, Bourguet W, Balaguer P, Zalko D. Characterization of novel ligands of ERα, ERβ, and PPARγ: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci. 2011; 122(2):372–382. PMID: 21622942.
Article34. Iasinskaia IM, Rozanov A. Effect of nonsteroidal estrogen-like substances on aromatase activity. Ukr Biokhim Zh. 2001; 73(3):121–125. PMID: 12035542.35. Mussi P, Ciana P, Raviscioni M, Villa R, Regondi S, Agradi E, Maggi A, Di Lorenzo D. Activation of brain estrogen receptors in mice lactating from mothers exposed to DDT. Brain Res Bull. 2005; 65(3):241–247. PMID: 15811587.
Article36. You L, Sar M, Bartolucci E, Ploch S, Whitt M. Induction of hepatic aromatase by p,p'-DDE in adult male rats. Mol Cell Endocrinol. 2001; 178(1-2):207–214. PMID: 11403911.37. Xu Y, Yu RM, Zhang X, Murphy MB, Giesy JP, Lam MH, Lam PK, Wu RS, Yu H. Effects of PCBs and MeSO2-PCBs on adrenocortical steroidogenesis in H295R human adrenocortical carcinoma cells. Chemosphere. 2006; 63(5):772–784. PMID: 16216300.38. Ulbrich B, Stahlmann R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol. 2004; 78(5):252–268. PMID: 15064922.
Article39. Kim YH, Kim SH, Lee HW, Chae HD, Kim CH, Kang BM. Increased viability of endometrial cells by in vitro treatment with di-(2-ethylhexyl) phthalate. Fertil Steril. 2010; 94(6):2413–2416. PMID: 20493477.
Article40. Ghisari M, Bonefeld-Jorgensen EC. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett. 2009; 189(1):67–77. PMID: 19463926.
Article41. Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ, Tyler CR. A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans R Soc Lond B Biol Sci. 2009; 364(1526):2047–2062. PMID: 19528055.
Article42. Ohtani H, Miura I, Ichikawa Y. Effects of dibutyl phthalate as an environmental endocrine disruptor on gonadal sex differentiation of genetic males of the frog Rana rugosa. Environ Health Perspect. 2000; 108(12):1189–1193. PMID: 11133400.
Article43. Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005; 113(8):1056–1061. PMID: 16079079.
Article44. Erkekoglu P, Giray B, Rachidi W, Hininger-Favier I, Roussel AM, Favier A, Hincal F. Effects of di(2-ethylhexyl)phthalate on testicular oxidant/antioxidant status in selenium-deficient and selenium-supplemented rats. Environ Toxicol. 2011.
Article45. Herr C, zur Nieden A, Koch HM, Schuppe HC, Fieber C, Angerer J, Eikmann T, Stilianakis NI. Urinary di(2-ethylhexyl)phthalate (DEHP) - Metabolites and male human markers of reproductive function. Int J Hyg Environ Health. 2009; 212(6):648–653. PMID: 19733116.46. Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl)phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001; 172(3):217–224. PMID: 11312650.47. Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003; 111(2):139–145. PMID: 12573895.
Article48. Stevens RG, Rea MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control. 2001; 12(3):279–287. PMID: 11405333.49. Choi SM, Yoo SD, Lee BM. Toxicological characteristics of endocrine-disrupting chemicals: developmental toxicity, carcinogenicity, and mutagenicity. J Toxicol Environ Health B Crit Rev. 2004; 7(1):1–24. PMID: 14681080.
Article50. Negoita M, Mihailovici MS. Expression of hormonal receptors (α-estrogen, β-estrogen, progesteron), Ki-67 and P53 in endometrium of tamoxifen treated breast cancer patients. Rev Med Chir Soc Med Nat Iasi. 2011; 115(3):834–838. PMID: 22046795.51. Swart JC, Pool EJ. Development of a bio-assay for estrogens using estrogen receptor alpha gene expression by MCF7 cells as biomarker. J Immunoassay Immunochem. 2009; 30(2):150–165. PMID: 19330641.
Article52. Berckmans P, Leppens H, Vangenechten C, Witters H. Screening of endocrine disrupting chemicals with MELN cells, an ER-transactivation assay combined with cytotoxicity assessment. Toxicol In Vitro. 2007; 21(7):1262–1267. PMID: 17572059.
Article53. Titus-Ernstoff L, Hatch EE, Hoover RN, Palmer J, Greenberg ER, Ricker W, Kaufman R, Noller K, Herbst AL, Colton T, Hartge P. Long-term cancer risk in women given diethylstilbestrol (DES) during pregnancy. Br J Cancer. 2001; 84(1):126–133. PMID: 11139327.
Article54. Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010; 1(3):146–155. PMID: 21761357.55. Tanaka T, Kohno H, Suzuki R, Sugie S. Lack of modifying effects of an estrogenic compound atrazine on 7,12-dimethylbenz(a)anthracene-induced ovarian carcinogenesis in rats. Cancer Lett. 2004; 210(2):129–137. PMID: 15183528.
Article56. Attia DM, Ederveen AG. Opposing roles of ERα and ERβ in the genesis and progression of adenocarcinoma in the rat ventral prostate. Prostate. 2011; in press.
Article57. Shah S, Hess-Wilson JK, Webb S, Daly H, Godoy-Tundidor S, Kim J, Boldison J, Daaka Y, Knudsen KE. 2,2-bis(4-chlorophenyl)-1,1-dichloroethylene stimulates androgen independence in prostate cancer cells through combinatorial activation of mutant androgen receptor and mitogen-activated protein kinase pathways. Mol Cancer Res. 2008; 6(9):1507–1520. PMID: 18819937.
Article58. Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab. 2011; 6(3):437–451. PMID: 21765856.
Article59. Wadia PR, Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Perspect. 2007; 115(4):592–598. PMID: 17450229.
Article60. Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Munoz-de-Toro M. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007; 115(1):80–86. PMID: 17366824.
Article61. Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007; 23(3):383–390. PMID: 17123778.
Article62. Jenkins S, Raghuraman N, Eltoum I, Carpenter M, Russo J, Lamartiniere CA. Oral exposure to bisphenol a increases dimethylbenzanthracene-induced mammary cancer in rats. Environ Health Perspect. 2009; 117(6):910–915. PMID: 19590682.
Article63. Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, Soto AM. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008; 26(3-4):210–219. PMID: 18938238.
Article64. Long X, Burke KA, Bigsby RM, Nephew KP. Effects of the xenoestrogen bisphenol A on expression of vascular endothelial growth factor (VEGF) in the rat. Exp Biol Med (Maywood). 2001; 226(5):477–483. PMID: 11393178.
Article65. Buteau-Lozano H, Velasco G, Cristofari M, Balaguer P, Perrot-Applanat M. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J Endocrinol. 2008; 196(2):399–412. PMID: 18252963.
Article66. Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Sci. 2002; 67(1):63–74. PMID: 11961217.67. Lewis BC, Hudgins S, Lewis A, Schorr K, Sommer R, Peterson RE, Flaws JA, Furth PA. In utero and lactational treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs mammary gland differentiation but does not block the response to exogenous estrogen in the postpubertal female rat. Toxicol Sci. 2001; 62(1):46–53. PMID: 11399792.68. Chow LW, Cheung MN, Loo WT, Guan XY. A rat cell line derived from DMBA-induced mammary carcinoma. Life Sci. 2003; 73(1):27–40. PMID: 12726884.
Article69. La Merrill M, Kuruvilla BS, Pomp D, Birnbaum LS, Threadgill DW. Dietary fat alters body composition, mammary development, and cytochrome p450 induction after maternal TCDD exposure in DBA/2J mice with low-responsive aryl hydrocarbon receptors. Environ Health Perspect. 2009; 117(9):1414–1419. PMID: 19750107.
Article70. Melnick RL. Is peroxisome proliferation an obligatory precursor step in the carcinogenicity of di(2-ethylhexyl) phthalate (DEHP)? Environ Health Perspect. 2001; 109(5):437–442. PMID: 11401753.71. Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol. 2006; 36(5):459–479. PMID: 16954067.
Article72. Xu C, Chen JA, Qiu Z, Zhao Q, Luo J, Yang L, Zeng H, Huang Y, Zhang L, Cao J, Shu W. Ovotoxicity and PPAR-mediated aromatase downregulation in female Sprague-Dawley rats following combined oral exposure to benzo[a]pyrene and di-(2-ethylhexyl) phthalate. Toxicol Lett. 2010; 199(3):323–332. PMID: 20920559.
Article73. Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, Huttner K, Hu H. Use of di(2-ethylhexyl)phthalate-containing medical products and urinary levels of mono(2-ethylhexyl)phthalate in neonatal intensive care unit infants. Environ Health Perspect. 2005; 113(9):1222–1225. PMID: 16140631.74. Mersereau JE, Levy N, Staub RE, Baggett S, Zogovic T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor â agonist. Mol Cell Endocrinol. 2008; 283(1-2):49–57. PMID: 18177995.
Article75. Levenson AS, Gehm BD, Pearce ST, Horiguchi J, Simons LA, Ward JE 3rd, Jameson JL, Jordan VC. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ERα. Int J Cancer. 2003; 104(5):587–596. PMID: 12594813.76. Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, Xiao Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007; 14(12):806–814. PMID: 17689939.
Article77. Meng QS, Zhu XY, Tang XL, Ma B, Ni X. Effect of isoflavones in regulating the transcription of target genes through estrogen receptors. Zhong Xi Yi Jie He Xue Bao. 2007; 5(5):577–580. PMID: 17854564.
Article78. Ye L, Chan MY, Leung LK. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol Cell Endocrinol. 2009; 302(1):73–80. PMID: 19356625.
Article79. Mai Z, Blackburn GL, Zhou JR. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol Carcinog. 2007; 46(7):534–542. PMID: 17295235.
Article80. Rowell C, Carpenter DM, Lamartiniere CA. Chemoprevention of breast cancer, proteomic discovery of genistein action in the rat mammary gland. J Nutr. 2005; 135(12 Suppl):2953S–2959S. PMID: 16317154.
Article81. Brown NM, Lamartiniere CA. Genistein regulation of transforming growth factor-α, epidermal growth factor (EGF), and EGF receptor expression in the rat uterus and vagina. Cell Growth Differ. 2000; 11(5):255–260. PMID: 10845426.82. Brisdelli F, D'Andrea G, Bozzi A. Resveratrol: a natural polyphenol with multiple chemopreventive properties. Curr Drug Metab. 2009; 10(6):530–546. PMID: 19702538.83. Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β. Endocrinology. 2000; 141(10):3657–3667. PMID: 11014220.
Article84. Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3'-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006; 5(5):1335–1341. PMID: 16731767.
Article85. Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002; 62(17):4945–4954. PMID: 12208745.86. Provinciali M, Re F, Donnini A, Orlando F, Bartozzi B, Di Stasio G, Smorlesi A. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer. 2005; 115(1):36–45. PMID: 15688416.
Article87. Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001; 61(20):7456–7463. PMID: 11606380.88. Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002; 291(4):1001–1005. PMID: 11866465.89. Whitsett TG Jr, Lamartiniere CA. Genistein and resveratrol: mammary cancer chemoprevention and mechanisms of action in the rat. Expert Rev Anticancer Ther. 2006; 6(12):1699–1706. PMID: 17181483.90. Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008; 29(11):2162–2168. PMID: 18632754.91. Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006; 231(1):113–122. PMID: 16356836.92. Cooper RL, Kavlock RJ. Endocrine disruptors and reproductive development: a weight-of-evidence overview. J Endocrinol. 1997; 152(2):159–166. PMID: 9071972.
Article93. Safe SH. Endocrine disruptors and human health - Is there a problem? An update. Environ Health Perspect. 2000; 108(6):487–493. PMID: 10856020.94. Talsness CE, Kuriyama SN, Sterner-Kock A, Schnitker P, Grande SW, Shakibaei M, Andrade A, Grote K, Chahoud I. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ Health Perspect. 2008; 116(3):308–314. PMID: 18335096.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endocrine-disrupting Chemicals: Review of Toxicological Mechanisms Using Molecular Pathway Analysis
- Hidden link between endocrine-disrupting chemicals and pediatric obesity
- Childhood obesity and endocrine disrupting chemicals
- Epigenetic control of endocrine disrupting chemicals on gynecological disease: Focused on phthalates
- ffects of Endocrine-Disrupting Chemicals on Human Health