J Korean Soc Radiol.
2011 Jul;65(1):41-51. 10.3348/jksr.2011.65.1.41.
Mechanical Recanalization of Cerebral Artery Embolic Occlusion Using a Self-Expanding Stent: Experimental Analysis in Canine Model
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. sjkimjb@amc.seoul.kr
- 2Department of Radiology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 1443492
- DOI: http://doi.org/10.3348/jksr.2011.65.1.41
Abstract
- PURPOSE
To evaluate the feasibility of a self-expanding stent for acute embolic occlusion, and recanalization mechanism by histologic examination.
MATERIALS AND METHODS
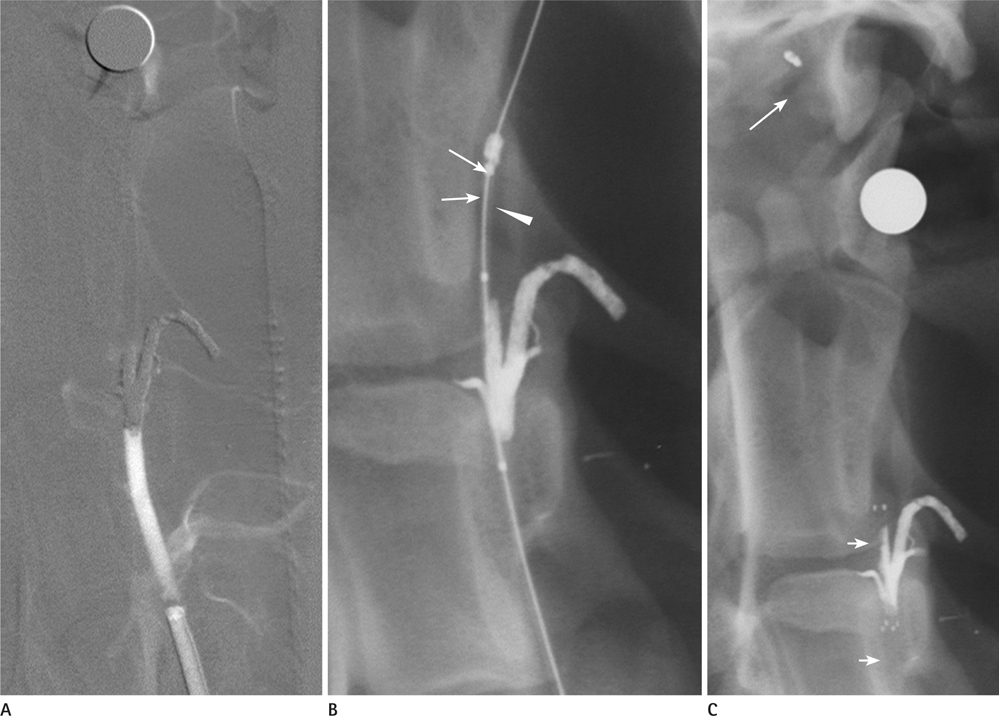
Five mongrel dogs were used as study subjects. Each vertebral artery was occluded, and a self-expanding stent was used for recanalization. We evaluated the technical success rate for the placement of the stent to the targeted vessel, the recanalization rate, and residual stenosis. We obtained two specimens of the stented vertebral arteries for histologic evaluation.
RESULTS
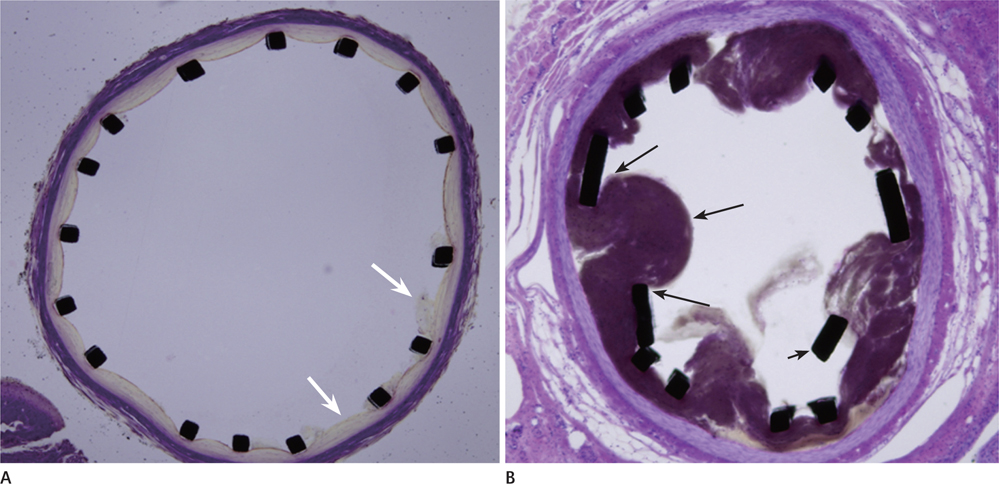
One dog died of an unknown cause during the induction of anesthesia. In two dogs, only one side of the vertebral artery was used, whereas both vertebral arteries were used in the remaining dogs. A total of six vertebral arteries were successfully occluded. The technical success rate for stenting without complication was 66.7%. The immediate recanalization rate after stenting was 100%. The residual stenosis was 35.6 +/- 18.6%. On microscopic examination, the stent concentrically displaced the clot and the clot was captured between the stent mesh and arterial wall.
CONCLUSION
Self-expanding stents were effective in revascularizing the cerebrovascular embolic occlusion. The self-expanding stent seemed to achieve recanalization by pushing the clot to the arterial wall and capturing the clot between the stent mesh and arterial wall.
MeSH Terms
Figure
Reference
-
1. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007; 38:967–973.2. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995; 333:1581–1587.3. Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005; 36:2311–2320.4. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999; 282:2003–2011.5. Ueda T, Sakaki S, Nochide I, Kumon Y, Kohno K, Ohta S. Angioplasty after intra-arterial thrombolysis for acute occlusion of intracranial arteries. Stroke. 1998; 29:2568–2574.6. Nakano S, Iseda T, Yoneyama T, Kawano H, Wakisaka S. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke. 2002; 33:2872–2876.7. Spreer J, Els T, Hetzel A, Arnold S, Klisch J, Huppertz HJ, et al. Primary stenting as emergency therapy in acute basilar artery occlusion. Neuroradiology. 2002; 44:791–795.8. Martinez H, Zoarski GH, Obuchowski AM, Stallmayer MJ, Papangelou A, Airan-Javia S. Mechanical thrombectomy of the internal carotid artery and middle cerebral arteries for acute stroke by using the retriever device. AJNR Am J Neuroradiol. 2004; 25:1812–1815.9. Noser EA, Shaltoni HM, Hall CE, Alexandrov AV, Garami Z, Cacayorin ED, et al. Aggressive mechanical clot disruption: a safe adjunct to thrombolytic therapy in acute stroke? Stroke. 2005; 36:292–296.10. Levy EI, Ecker RD, Horowitz MB, Gupta R, Hanel RA, Sauvageau E, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery. 2006; 58:458–463.11. Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol. 2006; 27:1177–1182.12. Choi JW, Kim JK, Choi BS, Kim JH, Hwang HJ, Kim JS, et al. Adjuvant revascularization of intracranial artery occlusion with angioplasty and/or stenting. Neuroradiology. 2009; 51:33–43.13. Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005; 36:1432–1438.14. Eberhardt O, Naegele T, Raygrotzki S, Weller M, Ernemann U. Stenting of vertebrobasilar arteries in symptomatic atherosclerotic disease and acute occlusion: case series and review of the literature. J Vasc Surg. 2006; 43:1145–1154.15. Flint AC, Duckwiler GR, Budzik RF, Liebeskind DS, Smith WS. MERCI and Multi MERCI Writing Committee. Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and Multi MERCI Part I trials. Stroke. 2007; 38:1274–1280.16. Gobin YP, Starkman S, Duckwiler GR, Grobelny T, Kidwell CS, Jahan R, et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke. 2004; 35:2848–2854.17. Fitzsimmons BF, Becske T, Nelson PK. Rapid stent-supported revascularization in acute ischemic stroke. AJNR Am J Neuroradiol. 2006; 27:1132–1134.18. Sauvageau E, Levy EI. Self-expanding stent-assisted middle cerebral artery recanalization: technical note. Neuroradiology. 2006; 48:405–408.19. Levy EI, Mehta R, Gupta R, Hanel RA, Chamczuk AJ, Fiorella D, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007; 28:816–822.20. Brekenfeld C, Schroth G, Mattle HP, Do DD, Remonda L, Mordasini P, et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke. 2009; 40:847–852.21. Kelly ME, Furlan AJ, Fiorella D. Recanalization of an acute middle cerebral artery occlusion using a self-expanding, reconstrainable, intracranial microstent as a temporary endovascular bypass. Stroke. 2008; 39:1770–1773.22. Howington JU, Hanel RA, Harrigan MR, Levy EI, Guterman LR, Hopkins LN. The Neuroform stent, the first microcatheter-delivered stent for use in the intracranial circulation. Neurosurgery. 2004; 54:2–5.23. Levy EI, Sauvageau E, Hanel RA, Parikh R, Hopkins LN. Self-expanding versus balloon-mounted stents for vessel recanalization following embolic occlusion in the canine model: technical feasibility study. AJNR Am J Neuroradiol. 2006; 27:2069–2072.24. Turk AS, Niemann DB, Ahmed A, Aagaard-Kienitz B. Use of self-expanding stents in distal small cerebral vessels. AJNR Am J Neuroradiol. 2007; 28:533–536.25. Chiam PT, Samuelson RM, Mocco J, Hanel RA, Siddiqui AH, Hopkins LN, et al. Navigability trumps all: stenting of acute middle cerebral artery occlusions with a new self-expandable stent. AJNR Am J Neuroradiol. 2008; 29:1956–1958.26. Hähnel S, Ringleb P, Hartmann M. Treatment of intracranial stenoses using the Neuroform stent system: initial experience in five cases. Neuroradiology. 2006; 48:479–485.27. Gralla J, Schroth G, Remonda L, Fleischmann A, Fandino J, Slotboom J, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol. 2006; 27:1357–1361.28. Gralla J, Burkhardt M, Schroth G, El-Koussy M, Reinert M, Nedeltchev K, et al. Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol. 2008; 29:247–252.29. Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008; 29:582–587.30. Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. IMS-I Investigators. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005; 36:2400–2403.31. Kaufman HH, Anderson JH, Huchton JD, Woo J. A new canine model of proximal internal carotid embolism. Stroke. 1979; 10:415–418.32. Qureshi AI, Boulos AS, Hanel RA, Suri MF, Yahia AM, Alberico RA, et al. Randomized comparison of intra-arterial and intravenous thrombolysis in a canine model of acute basilar artery thrombosis. Neuroradiology. 2004; 46:988–995.33. Ringer AJ, Guterman LR, Hopkins LN. Site-specific thromboembolism: a novel animal model for stroke. AJNR Am J Neuroradiol. 2004; 25:329–332.34. Brekenfeld C, Tinguely P, Schroth G, Arnold M, El-Koussy M, Nedeltchev K, et al. Percutaneous transluminal angioplasty and stent placement in acute vessel occlusion: evaluation of new methods for interventional stroke treatment. AJNR Am J Neuroradiol. 2009; 30:1165–1172.35. Suh DC, Sung KB, Cho YS, Choi CG, Lee HK, Lee JH, et al. Transluminal angioplasty for middle cerebral artery stenosis in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 1999; 20:553–558.36. Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009; 40:2761–2768.37. Grunwald IQ, Walter S, Papanagiotou P, Krick C, Hartmann K, Dautermann A, et al. Revascularization in acute ischaemic stroke using the penumbra system: the first single center experience. Eur J Neurol. 2009; 16:1210–1216.38. Mori T, Kazita K, Chokyu K, Mima T, Mori K. Short-term arteriographic and clinical outcome after cerebral angioplasty and stenting for intracranial vertebrobasilar and carotid atherosclerotic occlusive disease. AJNR Am J Neuroradiol. 2000; 21:249–254.39. Tsumoto T, Terada T, Tsuura M, Ryujin Y, Matsumoto H, Masuo O, et al. Endovascular therapy for acute thrombotic occlusion of the intracranial artery. Neuroradiology. 2004; 46:453–458.40. Suh DC, Lee SH, Kim KR, Park ST, Lim SM, Kim SJ, et al. Pattern of atherosclerotic carotid stenosis in Korean patients with stroke: different involvement of intracranial versus extracranial vessels. AJNR Am J Neuroradiol. 2003; 24:239–244.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Critical Use of Balloon Angioplasty after Recanalization Failure with Retrievable Stent in Acute Cerebral Artery Occlusion
- The Limitations of Thrombectomy with Solitaire(TM) AB as First-line Treatment in Acute Ischemic Stroke: A Single Center Experience
- Clinical and radiological outcomes of mechanical thrombectomy in simultaneous anterior cerebral artery and middle cerebral artery occlusion
- Angiographic and Clinical Factors Related with Good Functional Outcome after Mechanical Thrombectomy in Acute Cerebral Artery Occlusion
- How to Escape Stentriever Wedging in an Open-cell Carotid Stent during Mechanical Thrombectomy for Tandem Cervical Internal Carotid Artery and Middle Cerebral Artery Occlusion