Korean Circ J.
2012 Jun;42(6):390-396. 10.4070/kcj.2012.42.6.390.
Magnetic Bionanoparticle Enhances Homing of Endothelial Progenitor Cells in Mouse Hindlimb Ischemia
- Affiliations
-
- 1Innovative Research Institute for Cell Therapy, Seoul National University Hospital, Seoul, Korea. nowkang@snu.ac.kr
- 2Division of Cardiology, Seoul National University Hospital, Seoul, Korea.
- 3School of Chemical and Biological Engineering, Institute of Bioengineering, Seoul National University, Seoul, Korea.
- KMID: 1433858
- DOI: http://doi.org/10.4070/kcj.2012.42.6.390
Abstract
- BACKGROUND AND OBJECTIVES
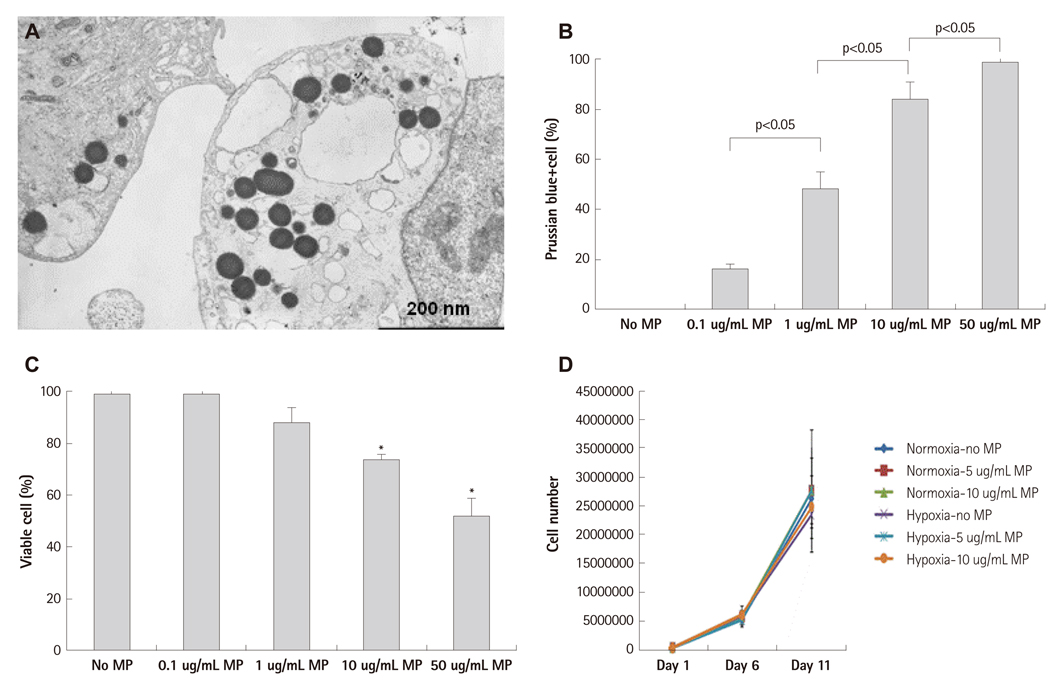
Poor homing efficiency is one of the major limitations of current stem cell therapy. Magnetic bionanoparticles (MPs) obtained from Magnetospirillum sp. AMB-1 have a lipid bilayer membrane and ferromagnetic properties. We evaluated a novel priming strategy using MPs to enhance the homing of transplanted progenitor cells to target tissue.
MATERIALS AND METHODS
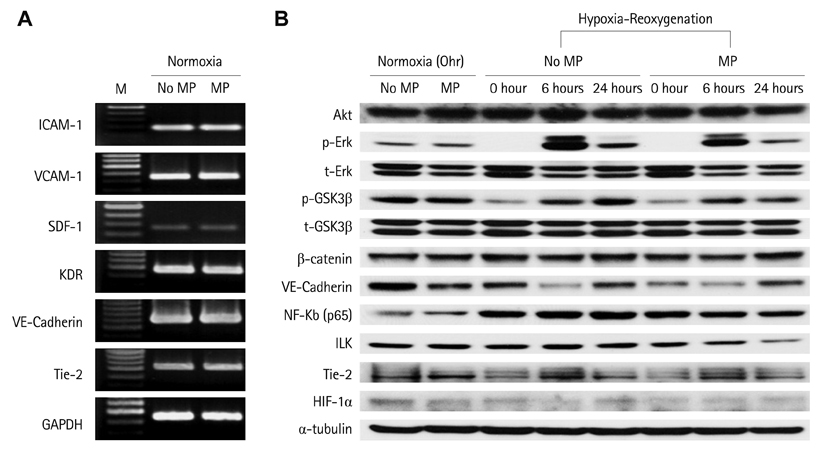
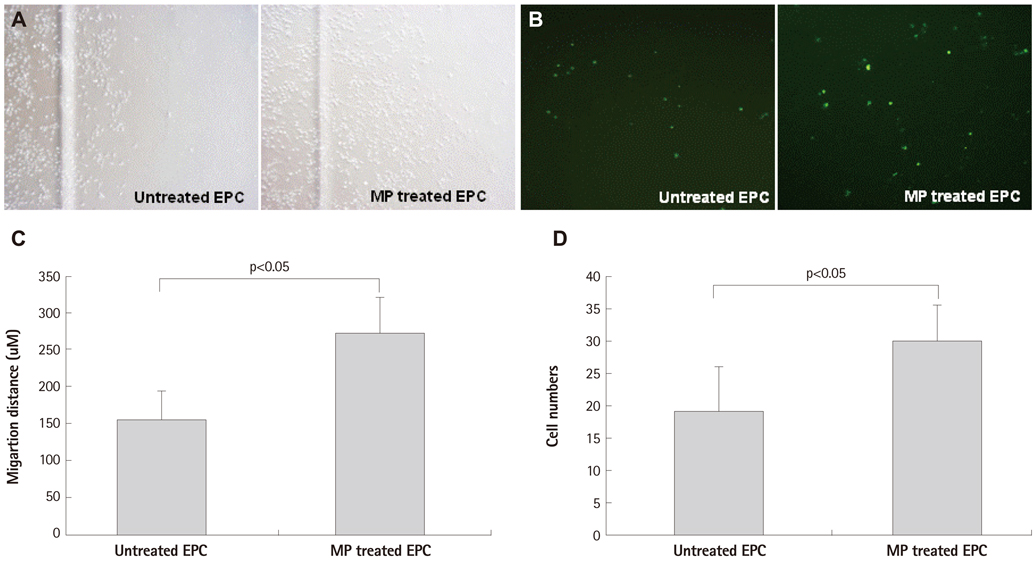
Effects of MP on proliferation, viability, and migration of late human endothelial progenitor cells (EPCs) were examined in vitro. Additionally, effects of MP on gene and protein expression related to survival and adhesion were evaluated. Homing and angiogenic efficiency of MP transferred late EPCs was evaluated in nude mouse hindlimb ischemia model.
RESULTS
Below threshold concentration, MP transfer did not influence proliferation or survival of late EPCs, but enhanced migration and trans-endothelial migration of late EPCs toward magnet. Below threshold concentration, MP transfer did not influence gene and protein expression related to survival. In the mouse hindlimb ischemia model, late EPCs treated with high dose MP (5 ug/mL) showed enhanced homing of injected late EPCs in the ischemic limb by magnet, compared to low dose MP (1 ug/mL) treated late EPCs. In addition, high dose MP transferred EPC showed significantly better improvement of perfusion in ischemic limb compared to untreated EPC.
CONCLUSION
MP transfer with magnet application can be a promising novel strategy to enhance homing efficacy and outcomes of current stem cell therapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The principles of tissue engineering and its recent advances and future prospects
Woo Seob Kim
J Korean Med Assoc. 2014;57(2):145-154. doi: 10.5124/jkma.2014.57.2.145.
Reference
-
1. Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006. 47:1295–1301.2. Templin C, Kotlarz D, Marquart F, et al. Transcoronary delivery of bone marrow cells to the infarcted murine myocardium: feasibility, cellular kinetics, and improvement in cardiac function. Basic Res Cardiol. 2006. 101:301–310.3. Agudelo CA, Tachibana Y, Noboru T, Iida H, Yamaoka T. Long-term in vivo magnetic resonance imaging tracking of endothelial progenitor cells transplanted in rat ischemic limbs and their angiogenic potential. Tissue Eng Part A. 2011. 17:2079–2089.4. Kim JA, Lee HJ, Kang HJ, Park TH. The targeting of endothelial progenitor cells to a specific location within a microfluidic channel using mag-netic nanoparticles. Biomed Microdevices. 2009. 11:287–296.5. Chaudeurge A, Wilhelm C, Chen-Tournoux A, et al. Can magnetic targeting of magnetically labeled circulating cells optimize intramyocardial cell retention? Cell Transplant. 2011. [Epub ahead of print].6. Kim HS, Skurk C, Maatz H, et al. Akt/FOXO3a signaling modulates the endothelial stress response through regulation of heat shock protein 70 expression. FASEB J. 2005. 19:1042–1044.7. Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004. 24:288–293.8. Yoon CH, Hur J, Park KW, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and miatrix metalloproteinases. Circulation. 2005. 112:1618–1627.9. Hill JM, Dick AJ, Raman VK, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003. 108:1009–1014.10. Matuszewski L, Persigehl T, Wall A, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005. 235:155–161.11. Komeili A. Molecular mechanisms of magnetosome formation. Annu Rev Biochem. 2007. 76:351–366.12. Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005. 26:3995–4021.13. Lei Han , Li SY, Yong Yang, Zhao FM, Jie Huang , Jin Chang . Research on the structure and performance of bacterial magnetic nanoparticles. J Biomater Appl. 2008. 22:433–448.14. Kobayshi T, Ochi M, Yanada S, et al. Augmentation of degenerated human cartilage in vitro using magnetically labeled mesenchymal stem cells and an external magnetic device. Arthroscopy. 2009. 25:1435–1441.15. Balakumaran A, Pawelczyk E, Ren J, et al. Superparamagnetic iron oxide nanoparticles labeling of bone marrow stromal (mesenchymal) cells does not affect their "stemness". PLoS One. 2010. 5:e11462.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of Endothelial Progenitor Cells from Cord Blood by Ex Vivo Expansion
- Systemic injection of recombinant human erythropoietin after focal cerebral ischemia enhances oligodendroglial and endothelial progenitor cells in rat brain
- Role of Stromal Cell-Derived Factor-1 in Endothelial Progenitor Cell-Mediated Vascular Repair and Regeneration
- Angiogenesis and cell therapy
- The Differentiation of Pluripotent Stem Cells towards Endothelial Progenitor Cells – Potential Application in Pulmonary Arterial Hypertension