Korean J Ophthalmol.
2012 Oct;26(5):378-382. 10.3341/kjo.2012.26.5.378.
Effect of Amiloride to Retinal Toxicity Induced by Tissue Plasminogen Activator
- Affiliations
-
- 1Department of Ophthalmology, Kim's Eye Hospital, Konyang University College of Medicine, Seoul, Korea.
- 2Department of Ophthalmology, Kangbuk Samsung Medical Center, Seoul, Korea.
- 3Department of Ophthalmology, Noone Eye Hospital, Seoul, Korea.
- 4Jung In Eye Clinic, Seoul, Korea.
- 5R & D Center, Eyegene Inc., Seoul, Korea.
- 6Myung-Gok Eye Research Institute, Kim's Eye Hospital, Konyang University College of Medicine, Daejeon, Korea. joonhlee@konyang.ac.kr
- KMID: 1397489
- DOI: http://doi.org/10.3341/kjo.2012.26.5.378
Abstract
- PURPOSE
The effects of amiloride on cellular toxicity caused by tissue plasminogen activator (tPA) in mouse primary retinal cells were investigated.
METHODS
Primary retinal cell cultures were maintained using glial conditioned medium. Commercial tPA and L-arginine were added, and the level of cyclic guanosine monophosphate (cyclic-GMP) in the culture supernatant was assessed using an ELISA assay. We measured the cell viability of cultured retinal cells pretreated with three different concentrations of amiloride (1, 10, and 100 microm) in addition to commercial tPA or L-arginine treatment.
RESULTS
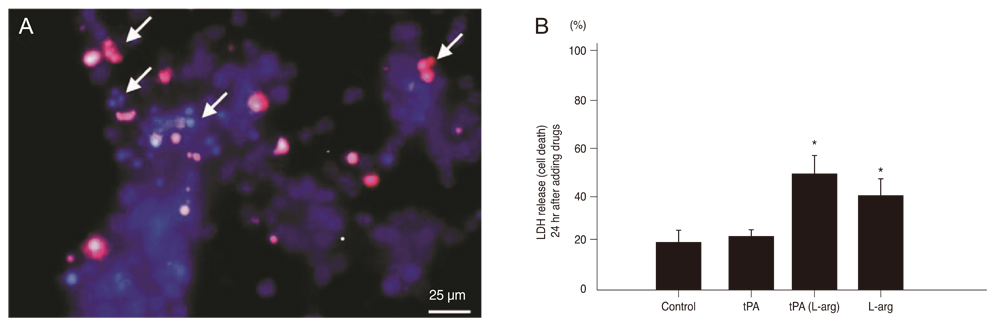
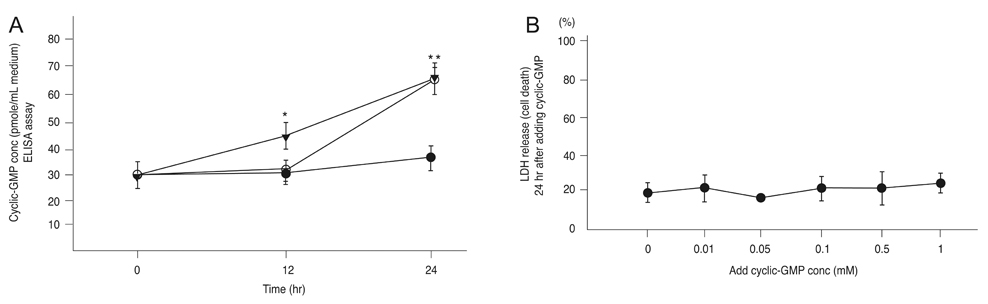
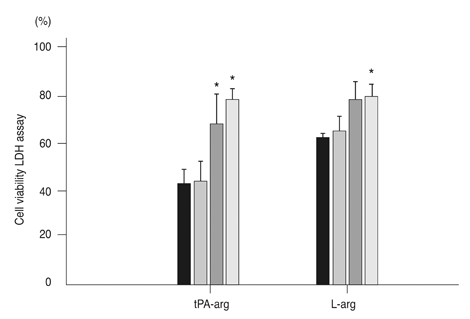
After exposing the cultured mouse retinal cells to tPA plus L-arginine or L-arginine alone, cyclic-GMP concentrations were 61.9 +/- 5.1 pmole/mL and 63.1 +/- 6.1 pmole/mL, respectively. However, the control group had a significantly lower concentration of cyclic-GMP (37.2 +/- 3.4 pmole/mL, p < 0.01). The cyclic GMP-dissolved solution did not cause retinal cell death. In the control group and the group treated with 1 microm amiloride and tPA containing L-arginine, the cell viability was 43.7% and 44.5%, respectively. However, cell viability increased to 70.6% with 10 microm amiloride and 78.4% with 100 microm amiloride (p = 0.015).
CONCLUSIONS
L-arginine increases intracellular cyclic-GMP and may give rise to retinal cells through this mechanism. In addition, amiloride in concentrations greater than 10 microm protects against L-arginine-induced retinal cell death.
MeSH Terms
Figure
Reference
-
1. Pennica D, Holmes WE, Kohr WJ, et al. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983. 301:214–221.2. Collen D, Stassen JM, Marafino BJ Jr, et al. Biological properties of human tissue-type plasminogen activator obtained by expression of recombinant DNA in mammalian cells. J Pharmacol Exp Ther. 1984. 231:146–152.3. Hassan AS, Johnson MW, Schneiderman TE, et al. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology. 1999. 106:1900–1906.4. Hattenbach LO, Klais C, Koch FH, Gumbel HO. Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology. 2001. 108:1485–1492.5. Peyman GA, Nelson NC Jr, Alturki W, et al. Tissue plasminogen activating factor assisted removal of subretinal hemorrhage. Ophthalmic Surg. 1991. 22:575–582.6. Kamei M, Tano Y, Maeno T, et al. Surgical removal of submacular hemorrhage using tissue plasminogen activator and perfluorocarbon liquid. Am J Ophthalmol. 1996. 121:267–275.7. Lewis H, VanderBrug Medendorp S. Tissue plasminogen activator-assisted surgical excision of subfoveal choroidal neovascularization in age-related macular degeneration: a randomized, double-masked trial. Ophthalmology. 1997. 104:1847–1851.8. Haupert CL, McCuen BW 2nd, Jaffe GJ, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001. 131:208–215.9. Johnson MW, Olsen KR, Hernandez E, et al. Retinal toxicity of recombinant tissue plasminogen activator in the rabbit. Arch Ophthalmol. 1990. 108:259–263.10. Oh HS, Kwon OW, Chung I, et al. Retinal toxicity of commercial tissue plasminogen activator is mediated by the induction of nitric oxide in the mouse retinal primary cells. Curr Eye Res. 2005. 30:291–297.11. Moncada S, Palmer RM. Biosynthesis and actions of nitric oxide. Semin Perinatol. 1991. 15:16–19.12. Rohrer B, Pinto FR, Hulse KE, et al. Multidestructive pathways triggered in photoreceptor cell death of the rd mouse as determined through gene expression profiling. J Biol Chem. 2004. 279:41903–41910.13. McLatchie LM, Matthews HR. The effect of pH on the block by L-cis-diltiazem and amiloride of the cyclic GMP-activated conductance of salamander rods. Proc Biol Sci. 1994. 255:231–236.14. Ho AK, Lalh SS, Young I, et al. Inhibitory effects of amilorides on pinealocyte adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate accumulation: possible involvement of postreceptor mechanisms. Endocrinology. 1990. 127:460–466.15. Shimizu S, Eguchi Y, Kamiike W, et al. Induction of apoptosis as well as necrosis by hypoxia and predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer Res. 1996. 56:2161–2166.16. Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987. 20:83–90.17. Moncada S, Palmer RM, Higgs EA. Biosynthesis of nitric oxide from L-arginine: a pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989. 38:1709–1715.18. Kurenny DE, Moroz LL, Turner RW, et al. Modulation of ion channels in rod photoreceptors by nitric oxide. Neuron. 1994. 13:315–324.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of immune-mediated vascular injury on the coagulation- regulatory mechanism of the human endothelial cells; changes of tissue-type plasminogen activator, plasminogen activator inhibitor- 1 and von Willebrand factor
- Resolution of experimental intravitreal fibrin by tissue plasminogen activator
- Retinal Toxicity of Intravitreal Tissue Plasminogen Activator on Submacular Hemorrhage
- Treatment of experimental vitreous hemorrhage with tissue plasminogen activator
- Effects of the dosing regimen of tissue-type plasminogen activator on blood coagulation system in experimental pulmonary embolism