Korean J Radiol.
2012 Oct;13(5):594-601. 10.3348/kjr.2012.13.5.594.
Comparison of MRI T2 Relaxation Changes of Knee Articular Cartilage before and after Running between Young and Old Amateur Athletes
- Affiliations
-
- 1Department of Radiology, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon 420-767, Korea. mj4907@schmc.ac.kr
- 2Department of Orthopedics, Soonchunhyang University College of Medicine, Seoul Hospital, Seoul 140-743, Korea.
- 3Department of Radiology, Soonchunhyang University College of Medicine, Seoul Hospital, Seoul 140-743, Korea.
- 4Department of Radiology, Soonchunhyang University College of Medicine, Cheonan Hospital, Cheonan 330-721, Korea.
- 5Department of Statistics, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea.
- 6Division of Rheumatology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon 420-767, Korea.
- KMID: 1392938
- DOI: http://doi.org/10.3348/kjr.2012.13.5.594
Abstract
OBJECTIVE
To compare changes in T2 relaxation on magnetic resonance (MR) images of knee articular cartilage in younger and older amateur athletes before and after running.
MATERIALS AND METHODS
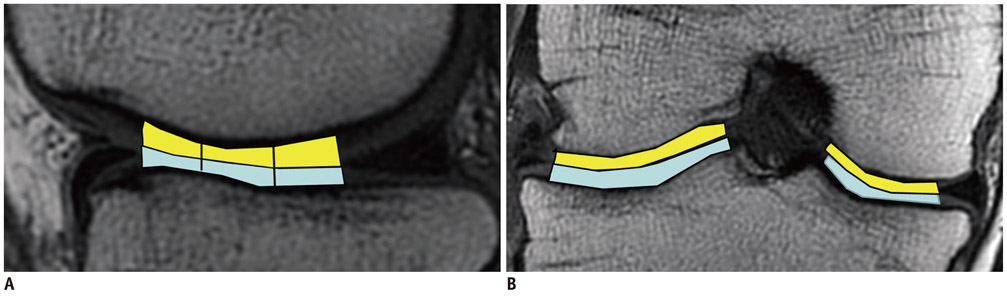
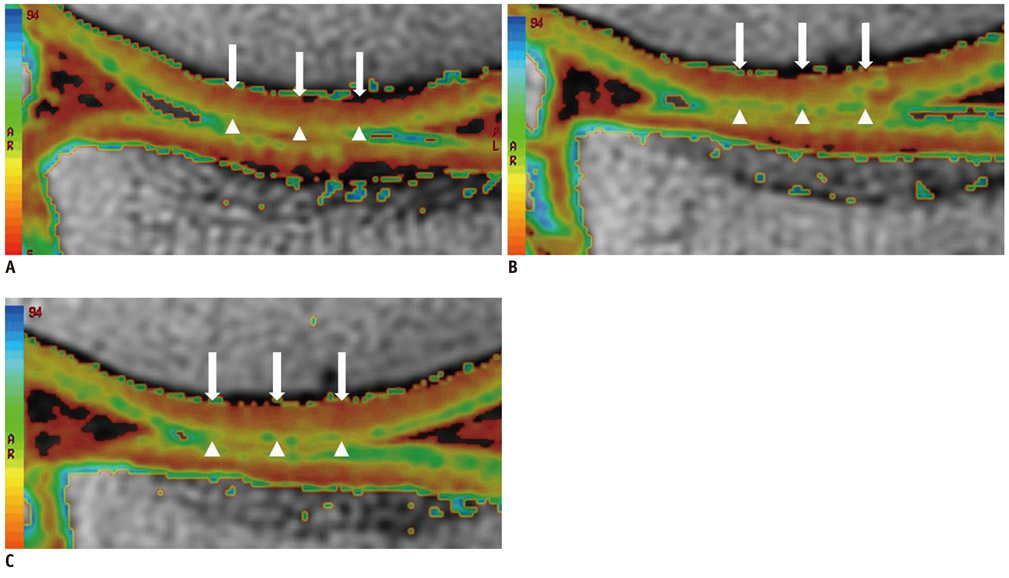
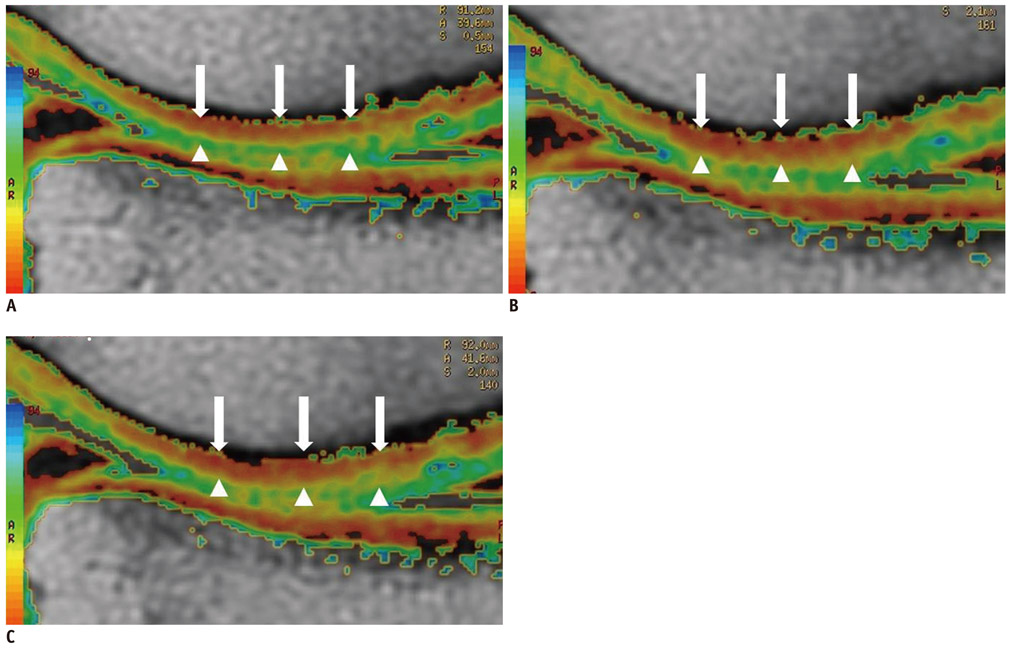
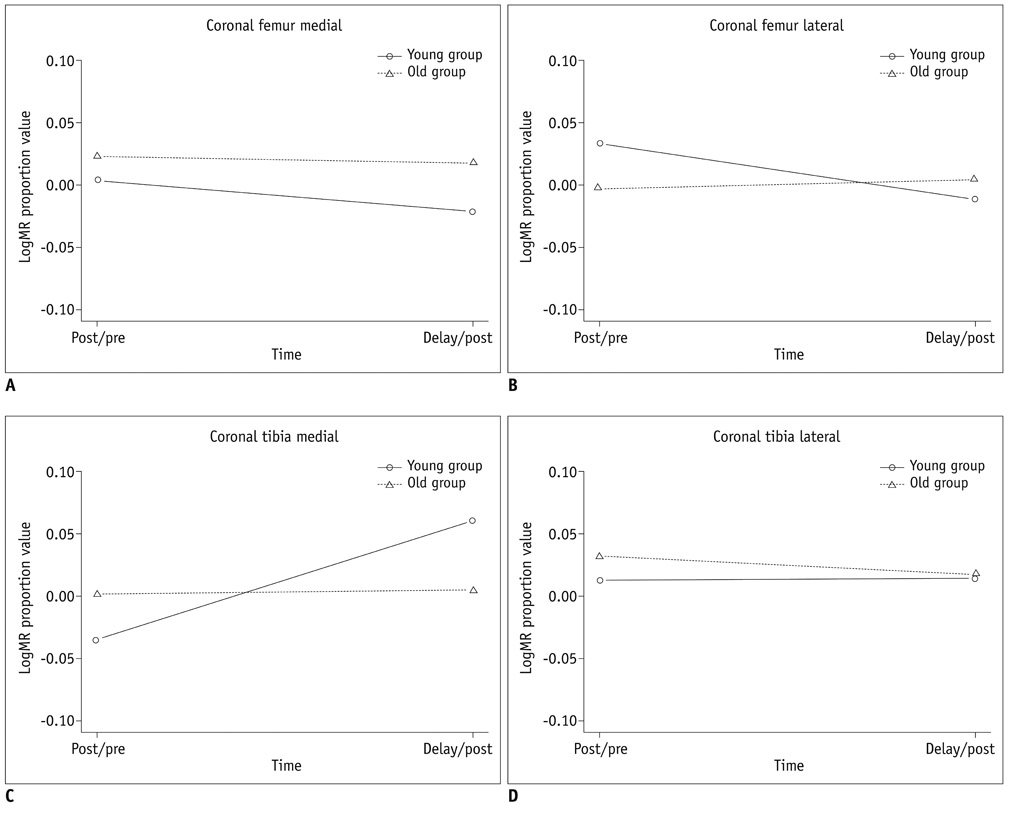
By using a 3.0-T MR imager, quantitative T2 maps of weight-bearing femoral and tibial articular cartilages in 10 younger and 10 older amateur athletes were acquired before, immediately after, and 2 hours after 30 minutes of running. Changes in global cartilage T2 signals of the medial and lateral condyles of the femur and tibia and regional cartilage T2 signals in the medial condyles of femoral and tibia in response to exercise were compared between the two age groups.
RESULTS
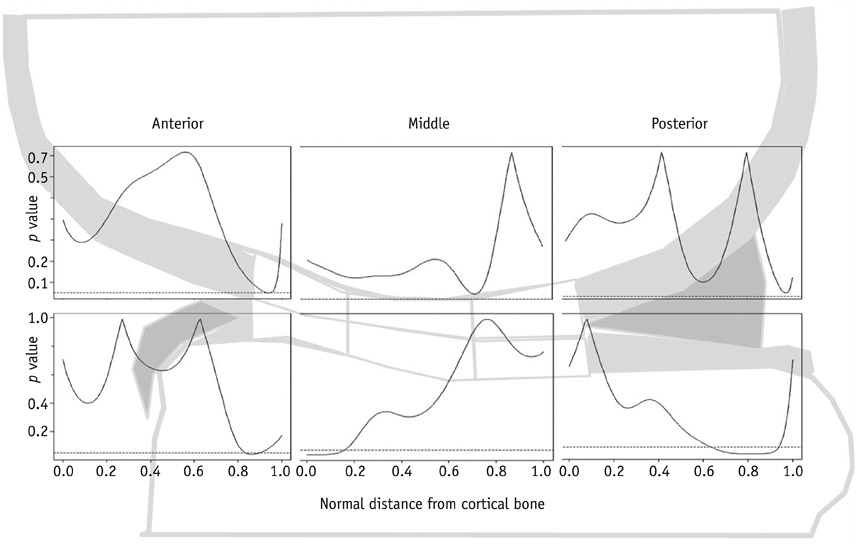
Changes in global cartilage T2 values after running did not differ significantly between the age groups. In terms of the depth variation, relatively higher T2 values in the older group than in the younger group were observed mainly in the superficial layers of the femoral and tibial cartilage (p < 0.05).
CONCLUSION
Age-related cartilage changes may occur mainly in the superficial layer of cartilage where collagen matrix degeneration is primarily initiated. However, no trend is observed regarding a global T2 changes between the younger and older age groups in response to exercise.
Keyword
MeSH Terms
Figure
Reference
-
1. Lane NE, Buckwalter JA. Exercise and osteoarthritis. Curr Opin Rheumatol. 1999. 11:413–416.2. Cymet TC, Sinkov V. Does long-distance running cause osteoarthritis? J Am Osteopath Assoc. 2006. 106:342–345.3. Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000. 35:622–638.4. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006. 241:407–414.5. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004. 8:355–368.6. Glaser C. New techniques for cartilage imaging: T2 relaxation time and diffusion-weighted MR imaging. Radiol Clin North Am. 2005. 43:641–653.7. Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011. 31:37–61.8. Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010. 254:509–520.9. Mamisch TC, Trattnig S, Quirbach S, Marlovits S, White LM, Welsch GH. Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading--initial results. Radiology. 2010. 254:818–826.10. Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005. 234:245–249.11. Csintalan RP, Schulz MM, Woo J, McMahon PJ, Lee TQ. Gender differences in patellofemoral joint biomechanics. Clin Orthop Relat Res. 2002. 260–269.12. Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004. 182:311–318.13. Van Breuseghem I. Ultrastructural MR imaging techniques of the knee articular cartilage: problems for routine clinical application. Eur Radiol. 2004. 14:184–192.14. Liess C, Lüsse S, Karger N, Heller M, Glüer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002. 10:907–913.15. Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol. 2001. 177:665–669.16. Gold GE, McCauley TR, Gray ML, Disler DG. What's new in cartilage? Radiographics. 2003. 23:1227–1242.17. Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010. 18:358–364.18. Nag D, Liney GP, Gillespie P, Sherman KP. Quantification of T(2) relaxation changes in articular cartilage with in situ mechanical loading of the knee. J Magn Reson Imaging. 2004. 19:317–322.19. Rubenstein JD, Kim JK, Henkelman RM. Effects of compression and recovery on bovine articular cartilage: appearance on MR images. Radiology. 1996. 201:843–850.20. Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995. 96:2859–2869.21. Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000. 214:259–266.22. Lüsse S, Claassen H, Gehrke T, Hassenpflug J, Schünke M, Heller M, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000. 18:423–430.23. Lüsse S, Knauss R, Werner A, Gründer W, Arnold K. Action of compression and cations on the proton and deuterium relaxation in cartilage. Magn Reson Med. 1995. 33:483–489.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Change of Articular Cartilage Thickness of the Knee Joint Related to Age in Korean
- Injuries and Illness during the 2019 Gwangju FINA and Masters World Championships in Elite and Amateur Athletes
- A Study on Osteoarthrosis in Korean Young Women Volley Ball Players
- Comparison of T1rho and T2 Mapping of Knee Articular Cartilage in an Asymptomatic Population
- Effect of Displacement and Morphological Change of Medial Meniscus on Early Osteoarthritis of the Knee