J Vet Sci.
2012 Sep;13(3):229-234. 10.4142/jvs.2012.13.3.229.
The effect of melanocortin (Mc3 and Mc4) antagonists on serotonin-induced food and water intake of broiler cockerels
- Affiliations
-
- 1Section of Physiology, Department of Basic Sciences, Faculty of Veterinary Medicine, University of Tehran, P.O. Box 14155-6453 Tehran, Iran. zendedel@ut.ac.ir
- 2Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Tehran, P.O. Box 14155-6453 Tehran, Iran.
- KMID: 1389761
- DOI: http://doi.org/10.4142/jvs.2012.13.3.229
Abstract
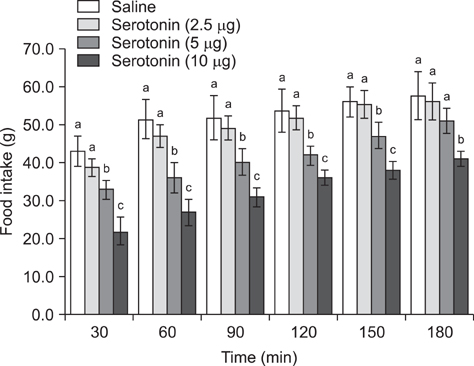
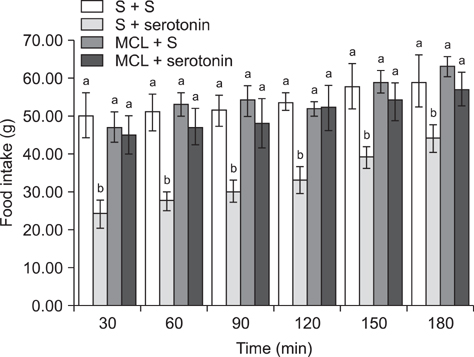
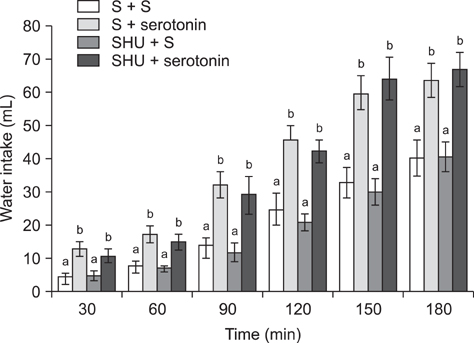
- The current study was designed to examine the effects of intracerebroventricular injections of SHU9119 [a nonselective melanocortin receptor (McR) antagonist] and MCL0020 (a selective McR antagonist) on the serotonin-induced eating and drinking responses of broiler cockerels deprived of food for 24 h (FD24). For Experiment 1, the chickens were intracerebroventricularly injected with 2.5, 5, and 10 microg serotonin. In Experiment 2, the chickens received 2 nmol SHU9119 before being injected with 10 microg serotonin. For Experiment 3, the chickens were given 10 microg serotonin after receiving 2 nmol MCL0020, and the level of food and water intake was determined 3 h post-injection. Results of this study showed that serotonin decreased food intake but increased water intake among the FD24 broiler cockerels and that these effects occurred in a dose-dependent manner. The inhibitory effect of serotonin on food intake was significantly attenuated by pretreatment with SHU9119 and MCL0020. However, the stimulatory effect of serotonin on water intake was not altered by this pretreatment. These results suggest that serotonin hypophagia and hyperdipsia were mediated by different mechanisms in the central nervous system, and that serotonin required downstream activation of McRs to promote hypophagia but not hyperdipsia in the FD24 chickens.
Keyword
MeSH Terms
-
Animals
Chickens
Dose-Response Relationship, Drug
Drinking Behavior/*drug effects
Feeding Behavior/*drug effects
Food Deprivation
Injections, Intraventricular/veterinary
Male
Melanocyte-Stimulating Hormones/*pharmacology
Oligopeptides/*pharmacology
Receptor, Melanocortin, Type 3/*antagonists & inhibitors
Receptor, Melanocortin, Type 4/*antagonists & inhibitors
Serotonin/pharmacology
Figure
Reference
-
1. Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LHT. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000. 26:97–102.
Article2. Denbow DM. Body temperature and food intake of turkeys following ICV injections of serotonin. Nutr Behav. 1983. 1:301–308.3. Denbow DM, Cherry JA, Siegel PB, Van Krey HP. Eating, drinking and temperature response of chicks to brain catecholamine injections. Physiol Behav. 1981. 27:265–269.
Article4. Denbow DM, Van Krey HP, Lacy MP, Dietrick TJ. Feeding, drinking and body temperature of Leghorn chicks: effects of ICV injections of biogenic amines. Physiol Behav. 1983. 31:85–90.
Article5. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997. 385:165–168.
Article6. Grill HJ, Donahey JCK, King L, Kaplan JM. Contribution of caudal brainstem to d-fenfluramine anorexia. Psychopharmacology (Berl). 1997. 130:375–381.7. Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science. 2002. 297:609–611.
Article8. Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006. 51:239–249.
Article9. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997. 88:131–141.
Article10. Kask A, Pähkla R, Irs A, Rägo L, Wikberg JES, Schiöth HB. Long-term administration of MC4 receptor antagonist HS014 causes hyperphagia and obesity in rats. Neuroreport. 1999. 10:707–711.
Article11. Kask A, Rägo L, Wikberg JES, Schiöth HB. Differential effects of melanocortin peptides on ingestive behaviour in rats: evidence against the involvement of MC3 receptor in the regulation of food intake. Neurosci Lett. 2000. 283:1–4.
Article12. Kawakami S, Bungo T, Ando R, Ohgushi A, Shimojo M, Masuda Y, Furuse M. Central administration of α-melanocyte stimulating hormone inhibits fasting- and neuropeptide Y-induced feeding in neonatal chicks. Eur J Pharmacol. 2000. 398:361–364.
Article13. Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003. 457:213–235.
Article14. Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998. 393:72–76.
Article15. Kuenzel WJ. Central neuroanatomical systems involved in the regulation of food intake in birds and mammals. J Nutr. 1994. 124:8 Suppl. 1355S–1370S.
Article16. Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998. 29:293–298.
Article17. Lee MD, Aloyo VJ, Fluharty SJ, Simansky KJ. Infusion of the serotonin1B (5-HT1B) agonist CP-93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacology (Berl). 1998. 136:304–307.
Article18. Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003. 23:7143–7154.
Article19. Ludwig DS, Mountjoy KG, Tatro JB, Gillette JA, Frederich RC, Flier JS, Maratos-Flier E. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am J Physiol. 1998. 274:E627–E633.20. Olanrewaju HA, Thaxton JP, Dozier WA 3rd, Purswell J, Roush WB, Branton SL. A review of lighting programs for broiler production. Int J Poult Sci. 2006. 5:301–308.21. Raffin-Sanson ML, Bertherat J. Mc3 and Mc4 receptors: complementary role in weight control. Eur J Endocrinol. 2001. 144:207–208.
Article22. Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004. 41:711–722.
Article23. Steffens SM, Casas DC, Milanez BC, Freitas CG, Paschoalini MA, Marino-Neto J. Hypophagic and dipsogenic effects of central 5-HT injections in pigeons. Brain Res Bull. 1997. 44:681–688.
Article24. Strader AD, Schiöth HB, Buntin JD. The role of the melanocortin system and the melanocortin-4 receptor in ring dove (Streptopelia risoria) feeding behavior. Brain Res. 2003. 960:112–121.
Article25. Tachibana T, Sugahara K, Ohgushi A, Ando R, Kawakami S, Yoshimatsu T, Furuse M. Intracerebroventricular injection of agouti-related protein attenuates the anorexigenic effect of alpha-melanocyte stimulating hormone in neonatal chicks. Neurosci Lett. 2001. 305:131–134.
Article26. Takeuchi S, Takahashi S. Melanocortin receptor genes in the chicken-tissue distributions. Gen Comp Endocrinol. 1998. 112:220–231.
Article27. Takeuchi S, Takahashi S. A possible involvement of melanocortin 3 receptor in the regulation of adrenal gland function in the chicken. Biochim Biophys Acta. 1999. 1448:512–518.
Article28. Takeuchi S, Teshigawara K, Takahashi S. Widespread expression of Agouti-related protein (AGRP) in the chicken: a possible involvement of AGRP in regulating peripheral melanocortin systems in the chicken. Biochim Biophys Acta. 2000. 1496:261–269.
Article29. Thurmon JC, Tranquilli WJ, Benson GJ, editors. Lumb and Jones' Veterinary Anesthesia. 1996. 3rd ed. Baltimore: Williams and Wilkins;686–735.30. Van Tienhoven A, Juhász LP. The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol. 1962. 118:185–197.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of pretreatment of serotonin synthesis inhibitor p-chlorophenylalanine on lipopolysaccharide-induced anorexia in rats
- A case of postoperative serotonin syndrome following the administration of fentanyl, palonosetron, and meperidine: A case report
- Adjunctive Lurasidone Suppresses Food Intake and Weight Gain Associated with Olanzapine Administration in Rats
- Preventive Effect of Serotonergic Drugs on LPS-Induced Acute Anorexia in Rats
- Effect of brain angiotensin II receptor antagonists and antisense oligonucleotide on drinking and renal renin in rats