Korean J Ophthalmol.
2012 Aug;26(4):248-254. 10.3341/kjo.2012.26.4.248.
A Potential Role of Crystallin in the Vitreous Bodies of Rats after Ischemia-reperfusion Injury
- Affiliations
-
- 1Pureun Eye Clinic, Jeonju, Korea.
- 2Department of Ophthalmology, Wonkwang University School of Medicine, Iksan, Korea. ysyang@wonkwang.ac.kr
- KMID: 1387390
- DOI: http://doi.org/10.3341/kjo.2012.26.4.248
Abstract
- PURPOSE
Ischemia-reperfusion injury (I/R injury) is known not only to induce hypoxic and oxidative stress, but also to cause retinal degeneration in rats. Crystallins, known to inhibit the formation of reactive oxygen species, reduce apoptotic cell death. Our goal was to clarify not only the role of I/R injury-mediated crystallins, but also to evaluate the correlation of these compounds to anti-inflammation in the vitreous body.
METHODS
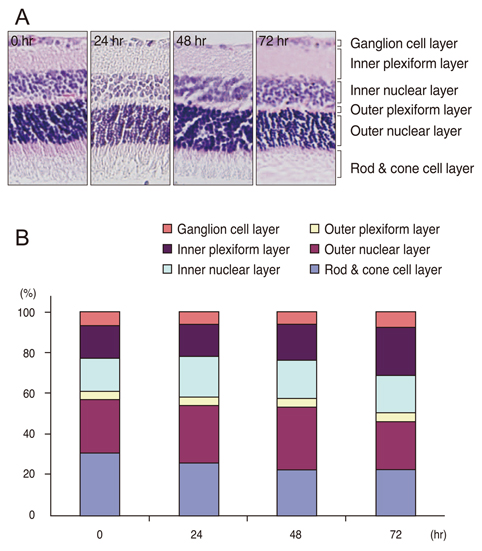
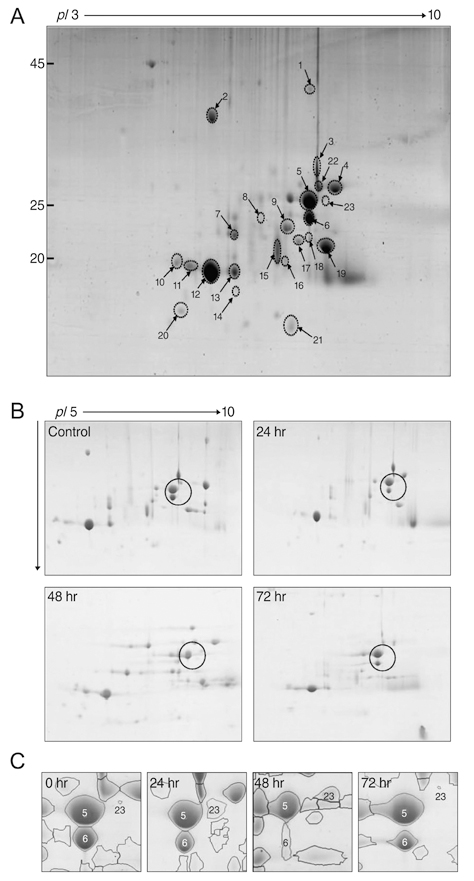
Twenty-four Sprague-Dawley rats were used in this study. We induced I/R injury by clamping the optic nerve for 30 minutes and then releasing it. The vitreous bodies were obtained from the experimental and control subjects 24, 48, and 72 hours after I/R injury. Two-dimensional electrophoresis was performed, and the targeted spots were further investigated using matrix-assisted laser desorption-ionization time-of-flight mass spectrometry, spectrophotometry, Western blotting, and histological examination.
RESULTS
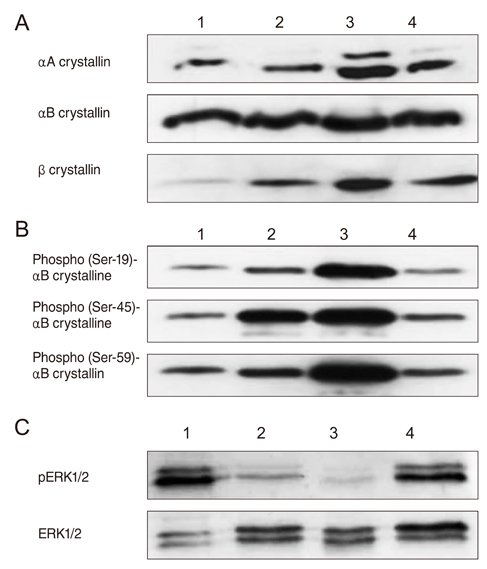
After I/R injury, 23 spots were identified as crystallins. The betaB2 crystallins were transcriptionally and post-translationally regulated, whereas the alphaB crystallins were controlled by post-translational modifications in the vitreous bodies of the rats. The total amounts of alphaA and beta crystallins (including isotypes of beta crystalline) had increased 48 hours after injury. The phosphorylation of alphaB crystallin (at serine residues 19, 45, and 59) was significantly increased 48 hours later, whereas phosphorylation of ERK1/2 showed the greatest decrease.
CONCLUSIONS
During hypoxic and oxidation stress, our results suggest that phosphorylated alphaB crystalline inhibits RAS, resulting in the inactivation of ERK1/2. The phosphorylation of alphaB crystallin may be associated with the inflammatory suppression in the vitreous body via the I/R injury model system.
MeSH Terms
Figure
Reference
-
1. White MY, Hambly BD, Jeremy RW, Cordwell SJ. Ischemia-specific phosphorylation and myofilament translocation of heat shock protein 27 precedes alpha B-crystallin and occurs independently of reactive oxygen species in rabbit myocardium. J Mol Cell Cardiol. 2006. 40:761–774.2. Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999. 79:609–634.3. Zimmerman BJ, Granger DN. Mechanisms of reperfusion injury. Am J Med Sci. 1994. 307:284–292.4. Wyllie AH. Apoptosis and the regulation of cell numbers in normal and neoplastic tissues: an overview. Cancer Metastasis Rev. 1992. 11:95–103.5. Abler AS, Chang CJ, Ful J, et al. Photic injury triggers apoptosis of photoreceptor cells. Res Commun Mol Pathol Pharmacol. 1996. 92:177–189.6. Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in alphaA-/alphaB-crystallin double-knockout mice. Development. 2006. 133:813–821.7. Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004. 11:512–526.8. Kantorow M, Piatigorsky J. Phosphorylations of alpha A- and alpha B-crystallin. Int J Biol Macromol. 1998. 22:307–314.9. Chiesa R, Gawinowicz-Kolks MA, Kleiman NJ, Spector A. The phosphorylation sites of the B2 chain of bovine alpha-crystallin. Biochem Biophys Res Commun. 1987. 144:1340–1347.10. Voorter CE, de Haard-Hoekman WA, Roersma ES, et al. The in vivo phosphorylation sites of bovine alpha B-crystallin. FEBS Lett. 1989. 259:50–52.11. Smith JB, Sun Y, Smith DL, Green B. Identification of the posttranslational modifications of bovine lens alpha B-crystallins by mass spectrometry. Protein Sci. 1992. 1:601–608.12. Martin JL, Mestril R, Hilal-Dandan R, et al. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997. 96:4343–4348.13. Bahk SC, Jang JU, Choi CU, et al. Post-translational modification of crystallins in vitreous body from experimental autoimmune uveitis of rats. J Proteome Res. 2007. 6:3891–3898.14. Bahk SC, Lee SH, Jang JU, et al. Identification of crystallin family proteins in vitreous body in rat endotoxin-induced uveitis: involvement of crystallin truncation in uveitis pathogenesis. Proteomics. 2006. 6:3436–3444.15. Schaefer H, Chamrad DC, Herrmann M, et al. Study of posttranslational modifications in lenticular alphaA-Crystallin of mice using proteomic analysis techniques. Biochim Biophys Acta. 2006. 1764:1948–1962.16. Scott JE. The chemical morphology of the vitreous. Eye (Lond). 1992. 6(Pt 6):553–555.17. Haddad A, De Almeida JC, Laicine EM, et al. The origin of the intrinsic glycoproteins of the rabbit vitreous body: an immunohistochemical and autoradiographic study. Exp Eye Res. 1990. 50:555–561.18. Haddad A, Laicine EM, de Almeida JC, Costa MS. Partial characterization, origin and turnover of glycoproteins of the rabbit vitreous body. Exp Eye Res. 1990. 51:139–143.19. Cho YM, Bae SH, Choi BK, et al. Differential expression of the liver proteome in senescence accelerated mice. Proteomics. 2003. 3:1883–1894.20. Choi BK, Chitwood DJ, Paik YK. Proteomic changes during disturbance of cholesterol metabolism by azacoprostane treatment in Caenorhabditis elegans. Mol Cell Proteomics. 2003. 2:1086–1095.21. Shevchenko A, Jensen ON, Podtelejnikov AV, et al. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci U S A. 1996. 93:14440–14445.22. Hoover HE, Thuerauf DJ, Martindale JJ, Glembotski CC. alpha B-crystallin gene induction and phosphorylation by MKK6-activated p38. A potential role for alpha B-crystallin as a target of the p38 branch of the cardiac stress response. J Biol Chem. 2000. 275:23825–23833.23. Li DW, Liu JP, Mao YW, et al. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell. 2005. 16:4437–4453.24. Liu JP, Schlosser R, Ma WY, et al. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004. 79:393–403.25. Inagaki N, Hayashi T, Arimura T, et al. Alpha B-crystallin mutation in dilated cardiomyopathy. Biochem Biophys Res Commun. 2006. 342:379–386.26. Fukushima K, Mizuno Y, Takatama M, Okamoto K. Increased neuronal expression of alpha B-crystallin in human olivary hypertrophy. Neuropathology. 2006. 26:196–200.27. Van Noort JM, Verbeek R, Meilof JF, et al. Autoantibodies against alpha B-crystallin, a candidate autoantigen in multiple sclerosis, are part of a normal human immune repertoire. Mult Scler. 2006. 12:287–293.28. Bhat SP, Nagineni CN. Alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989. 158:319–325.29. Ecroyd H, Meehan S, Horwitz J, et al. Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem J. 2007. 401:129–141.30. Huang Y, Shan J, Wang C, et al. Can ischemic preconditioning alone really protect organs from ischemia reperfusion injury in transplantation. Transpl Immunol. 2009. 20:127–131.31. Arai M, Thurman RG, Lemasters JJ. Ischemic preconditioning of rat livers against cold storage-reperfusion injury: role of nonparenchymal cells and the phenomenon of heterologous preconditioning. Liver Transpl. 2001. 7:292–299.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of CD 18 on Ischemia-Reperfusion Injury of TRAM Flap of Rats
- Effect of Selenium on Lung Injury Induced by Limb Ischemic Reperfusion Injury in Sprague-Dawley Rats
- Experimental Study on the Characteristics of Nerve Injury after Ischemia-Reperfusion and Their Recovery in Rats
- The Change of Histologic Features and Eicosanoids Level in Rat Testicular Ischemia-Reperfusion Injury
- The Effects of Glucocorticoid and alpha-Lipoic Acid on Ischemia-Reperfusion Injury