Korean J Radiol.
2008 Oct;9(5):432-438. 10.3348/kjr.2008.9.5.432.
A Meta-Analysis of the Accuracy of Prostate Cancer Studies Which Use Magnetic Resonance Spectroscopy as a Diagnostic Tool
- Affiliations
-
- 1The 2nd Affiliated Hospital of Medical School, Xi'an Jiao To, Imaging Center, China. yt.wangpeng813@163.com
- 2Beijing ChaoYang Hospital, Capital Medical University, Imaging Center, China.
- 3The 1st Affiliated Hospital of Medical School, Xi'an Jiao To, Imaging Center, China.
- 4Medical College of Xi'an Jiaotong University, Department of Health Statistics, China.
- KMID: 1385402
- DOI: http://doi.org/10.3348/kjr.2008.9.5.432
Abstract
OBJECTIVE
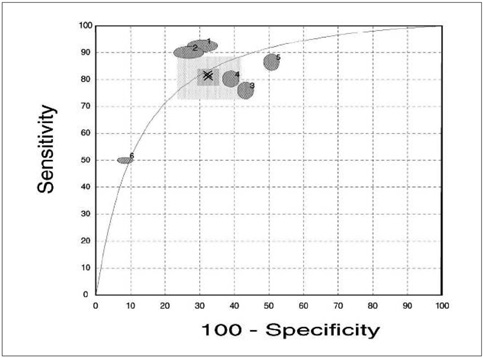
We aimed to do a meta-analysis of the existing literature to assess the accuracy of prostate cancer studies which use magnetic resonance spectroscopy (MRS) as a diagnostic tool. MATERIALS AND METHODS: Prospectively, independent, blind studies were selected from the Cochrane library, Pubmed, and other network databases. The criteria for inclusion and exclusion in this study referenced the criteria of diagnostic research published by the Cochrane center. The statistical analysis was adopted by using Meta-Test version 6.0. Using the homogeneity test, a statistical effect model was chosen to calculate different pooled weighted values of sensitivity, specificity, and the corresponding 95% confidence intervals (95% CI). The summary receiver operating characteristic (SROC) curves method was used to assess the results. RESULTS: We chose two cut-off values (0.75 and 0.86) as the diagnostic criteria for discriminating between benign and malignant. In the first diagnostic criterion, the pooled weighted sensitivity, specificity, and corresponding 95% CI (expressed as area under curve [AUC]) were 0.82 (0.73, 0.89), 0.68 (0.58, 0.76), and 83.4% (74.97, 91.83). In the second criterion, the pooled weighted sensitivity, specificity, and corresponding 95% CI were 0.64 (0.55, 0.72), 0.86 (0.79, 0.91) and 82.7% (68.73, 96.68). CONCLUSION: As a new method in the diagnostic of prostate cancer, MRS has a better applied value compared to other common modalities. Ultimately, large scale RCT (randomized controlled trial) randomized controlled trial studies are necessary to assess its clinical value.
Keyword
MeSH Terms
Figure
Reference
-
1. van Dorsten FA, van der Graaf M, Engelbrecht MR, van Leenders GJ, Verhofstad A, Rijpkema M, et al. Combined quantitative dynamic contrast-enhanced MR imaging and (1)H MR spectroscopic imaging of human prostate cancer. J Magn Reson Imaging. 2004. 20:279–287.2. Liu ZW, Xiang YB, Zhang W, Fang RR, Wan ZX, Sun L, et al. Incidence trends of prostate cancer in urban Shanghai (1973~1999). Chinese Journal of Health Statistics. 2003. 20:335–337.3. Scardino PT. The Gordon Wilson Lecture. Natural history and treatment of early stage prostate cancer. Trans Am Clin Climatol Assoc. 2000. 111:201–241.4. Carter HB, Partin AW. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Diagnosis and staging of prostate cancer. Campbell's urology. 1998. III:7th ed. Philadelphia: Saunders;2519–2537.5. Kurhanewicz J, Vigneron DB, Nelson SJ, Hricak H, MacDonald JM, Konety B, et al. Citrate as an in vivo marker to discriminate prostate cancer from benign prostatic hyperplasia and normal prostate peripheral zone: detection via localized proton spectroscopy. Urology. 1995. 45:459–466.6. Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Three-dimensional H-1 MR spectroscopy imaging of the in situ human prostate with high (0.24-0.7 cm) spatial resolution. Radiology. 1996. 198:795–805.7. Clark TJ, Mann CH, Shan N, Khan KS, Song F, Gupta JK. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial cancer: a systematic quantitative review. BJOG. 2002. 109:313–321.8. McAlister FA, Straus SE, Sackett DL. Why we need large, simple studies of the clinical examination: the problem and a proposed solution. Lancet. 1999. 354:1721–1724.9. Liu M, Guo YM, Guo XJ, Chen M, Zhang SJ. Evaluation of 99m Tc-MIBI scinti-mammorgraphy in the diagnosis of primary breast cancer: a meta-analysis. Chinese J Evidence-Based Medicine. 2005. 5:536–542.10. Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data analytic approaches and some additional considerations. Stat Med. 1993. 12:1293–1316.11. Mueller-Lisse UG, Vigneron DB, Hricak H, Swanson MG, Carroll PR, Bessette A, et al. Localized prostate cancer: effect of hormone deprivation therapy measured by using combined three-dimensional 1H MR spectroscopy and MR imaging: clinicopathologic case-controlled study. Radiology. 2001. 221:380–390.12. Jung JA, Coakley FV, Vigneron DB, Swanson MG, Qayyum A, Weinberg V, et al. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology. 2004. 233:701–708.13. Wefer AE, Hricak H, Vigneron DB, Coakley FV, Lu Y, Wefer J, et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol. 2000. 164:400–404.14. Scheidler J, Hricak H, Vigneron DB, Yu KK, Sokolov DL, Huang LR, et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging clinicopathologic study. Radiology. 1999. 213:473–480.15. Kyle KY, Juergen S, Hedvig H, Daniel BV, Charles JZ, Ryan GM, et al. Prostate cancer: prediction of extracapsular extentsion with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology. 1999. 213:481–488.16. Yuen JS, Thng CH, Tan PH, Khin LW, Phee SJ, Xiao D, et al. Endorectal magnetic resonance imaging and spectroscopy for the detection of tumor foci in men with prior negative transrectal ultrasound prostate biopsy. J Urol. 2004. 171:1482–1486.17. Prando A, Kurhanewicz J, Borges AP, Oliveira EM Jr, Figueiredo E. Prostatic biopsy directed with endorectal MR spectroscopic imaging findings in patients with elevated prostate specific antigen levels and prior negative biopsy findings: early experience. Radiology. 2005. 236:903–910.18. Liu GJ, Wu TX. Summary ROC curve-diagnostic test method of Meta-analysis. Chin J Evidence-Based Medicine. 2003. 3:41–44.19. Ikonen S, Kivisaari L, Tervahartiala P, Vehmas T, Taari K, Rannikko S. Prostate MR imaging. Accuracy in differentiating cancer from other prostatic disorders. Acta Radiol. 2001. 42:348–354.20. Hricak H, White S, Vigneron D, kurhanewicz J, Kosco A, Levin D, et al. Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal-pelvic phased-array coils. Radiology. 1994. 193:703–709.21. Jager GJ, Severens JL, Thornbury JR, de La Rosettee JJ, Ruijs SH, Barentsz JO. Prostate cancer staging: should MR imaging be used? A decision analytic approach. Radiology. 2000. 215:445–451.22. Filip GC, Hedvig H, Robert RH. Pretreatment evaluation of prostate cancer. Role of MR imaging and 1H MR spectroscopy. Radiographics. 2004. 24:167–180.23. Wefer AE, Hricak H, Vigneron DB, Coakley FV, Lu Y, Wefer J, et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol. 2000. 164:400–404.24. Cookson MS, Fleshner NE, Soloway SM, Fair WR. Correlation between Gleason score of needle biopsy and radical prostatectomy specimen: accuracy and clinical implications. J Urol. 1997. 157:559–562.25. Zakian KL, Sircar K, Hricak H, Chen HN, Shukla-Dave A, Eberhardt S, et al. Correlation of proton MR spectroscopic imaging with gleason score based on step-section pathologic analysis after radical prostatectomy. Radiology. 2005. 234:804–814.26. Engelbrecht MR, Jager GJ, Laheij RJ, Verbeek AL, van Lier HJ, Barentsz JO. Local staging of prostate cancer using magnetic resonance imaging: a meta-analysis. Eur Radiol. 2002. 12:2294–2302.27. Wang XY, Zhou LP, Ding JP, Li FY, Shan GZ, Xiao JX, et al. Quantitative criteria of MR spectroscopy in the differential diagnosis of prostate cancer in China: preliminary study. Chin J Med Imaging Technol. 2004. 20:1150–1153.28. Li Yan, Li JJ, Cen S, Li CZ, Yu N, Zhao YM, et al. Quantitative analysis of MR spectroscopy in diagnosis of prostate cancer. Chin J Med Imaging Technol. 2005. 21:1915–1917.29. Qin HY, Bai RJ, Wang XM, Zhao X. The value of three-dimensional proton MR spectroscopic imaging in the differential diagnosis of prostate cancer in peripheral zone: preliminary study. J Med Imaging. 2005. 15:978–982.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overview of the Process of Conducting Meta-analyses of the Diagnostic Test Accuracy
- Meta-Analysis of Diagnostic Test Accuracy
- Medical imaging of prostate cancer
- The Use of Magnetic Resonance Imaging in the Prostate Cancer Primary Diagnostic Pathway: Is It Ready for Primetime?
- Multidisciplinary Functional MR Imaging for Prostate Cancer