J Vet Sci.
2012 Mar;13(1):49-58. 10.4142/jvs.2012.13.1.49.
Cloning and characterization of a selenium-independent glutathione peroxidase (HC29) from adult Haemonchus contortus
- Affiliations
-
- 1College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, Jiangsu, China. lixiangrui@njau.edu.cn
- KMID: 1365003
- DOI: http://doi.org/10.4142/jvs.2012.13.1.49
Abstract
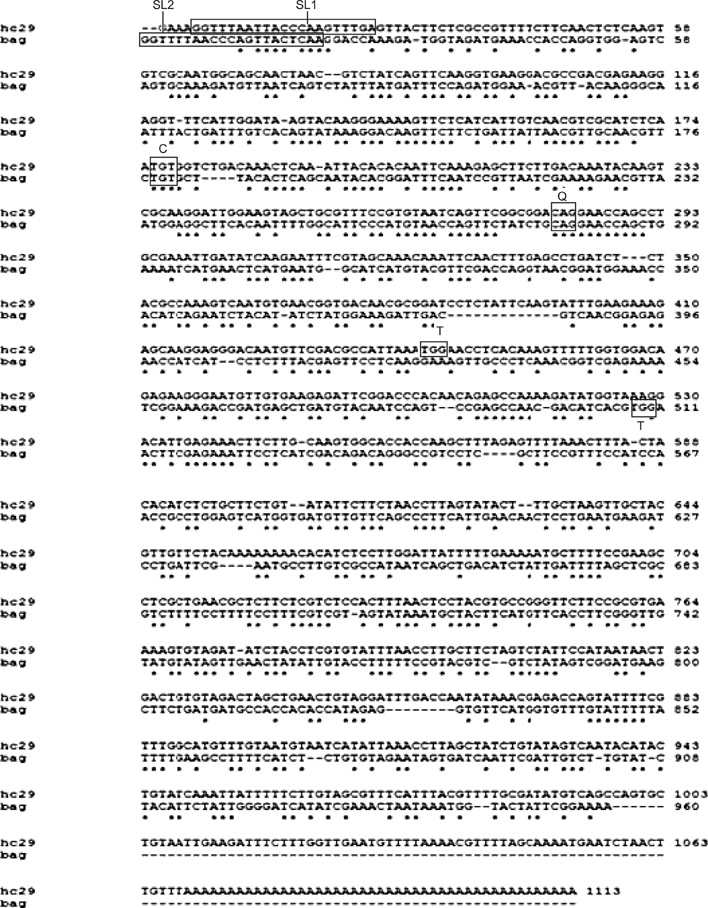
- The complete coding sequence of Haemonchus (H.) contortus HC29 cDNA was generated by rapid amplification of cDNA ends in combination with PCR using primers targeting the 5'- and 3'-ends of the partial mRNA sequence. The cloned HC29 cDNA was shown to be 1,113 bp in size with an open reading frame of 507 bp, encoding a protein of 168 amino acid with a calculated molecular mass of 18.9 kDa. Amino acid sequence analysis revealed that the cloned HC29 cDNA contained the conserved catalytic triad and dimer interface of selenium-independent glutathione peroxidase (GPX). Alignment of the predicted amino acid sequences demonstrated that the protein shared 44.7~80.4% similarity with GPX homologues in the thioredoxin-like family. Phylogenetic analysis revealed close evolutionary proximity of the GPX sequence to the counterpart sequences. These results suggest that HC29 cDNA is a GPX, a member of the thioredoxin-like family. Alignment of the nucleic acid and amino acid sequences of HC29 with those of the reported selenium-independent GPX of H. contortus showed that HC29 contained different types of spliced leader sequences as well as dimer interface sites, although the active sites of both were identical. Enzymatic analysis of recombinant prokaryotic HC29 protein showed activity for the hydrolysis of H2O2. These findings indicate that HC29 is a selenium-independent GPX of H. contortus.
MeSH Terms
-
Amino Acid Sequence
Animals
Base Sequence
Cloning, Molecular
DNA, Complementary/genetics/isolation & purification
Glutathione Peroxidase/*genetics/*metabolism
Goat Diseases/parasitology
Goats
Haemonchiasis/parasitology/prevention & control/*veterinary
Haemonchus/*enzymology/*genetics
Hydrogen Peroxide/metabolism
Molecular Sequence Data
Phylogeny
RNA, Helminth/chemistry/genetics
Random Amplified Polymorphic DNA Technique
Rats
Rats, Sprague-Dawley
Sequence Alignment
Sequence Analysis, DNA
Figure
Reference
-
1. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997. 25:3389–3402.
Article2. Bagnall NH, Kotze AC. cDNA cloning and expression patterns of a peroxiredoxin, a catalase and a glutathione peroxidase from Haemonchus contortus. Parasitol Res. 2004. 94:283–289.
Article3. Blaxter M, Liu L. Nematode spliced leaders-ubiquity, evolution and utility. Int J Parasitol. 1996. 26:1025–1033.4. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.
Article5. Callahan HL, Crouch RK, James ER. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today. 1988. 4:218–225.
Article6. Chiumiento L, Bruschi F. Enzymatic antioxidant systems in helminth parasites. Parasitol Res. 2009. 105:593–603.
Article7. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987. 162:156–159.
Article8. Cookson E, Tang L, Selkirk ME. Conservation of primary sequence of gp29, the major soluble cuticular glycoprotein, in three species of lymphatic filariae. Mol Biochem Parasitol. 1993. 58:155–159.
Article9. Cross AR, Jones OT. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991. 1057:281–298.
Article10. Dalton JP, Mulcahy G. Parasite vaccines-a reality? Vet Parasitol. 2001. 98:149–167.11. Falquet L, Pagni M, Bucher P, Hulo N, Sigrist CJA, Hofmann K, Bairoch A. The PROSITE database, its status in 2002. Nucleic Acids Res. 2002. 30:235–238.
Article12. Flohé L, Hecht HJ, Steinert P. Glutathione and trypanothione in parasitic hydroperoxide metabolism. Free Radic Biol Med. 1999. 27:966–984.
Article13. Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974. 104:580–587.
Article14. Hartman D, Donald DR, Nikolaou S, Savin KW, Hasse D, Presidente PJA, Newton SE. Analysis of developmentally regulated genes of the parasite Haemonchus contortus. Int J Parasitol. 2001. 31:1236–1245.
Article15. Henkle-Dührsen K, Kampkötter A. Antioxidant enzyme families in parasitic nematodes. Mol Biochem Parasitol. 2001. 114:129–142.
Article16. Knox DP, Redmond DL, Jones DG. Characterization of proteinases in extracts of adult Haemonchus contortus, the ovine abomasal nematode. Parasitology. 1993. 106(Pt 4):395–404.
Article17. Kuersten S, Lea K, MacMorris M, Spieth J, Blumenthal T. Relationship between 3' end formation and SL2-specific trans-splicing in polycistronic Caenorhabditis elegans pre-mRNA processing. RNA. 1997. 3:269–278.18. Lall S, Friedman CC, Jankowska-Anyszka M, Stepinski J, Darzynkiewicz E, Davis RE. Contribution of trans-splicing, 5'-leader length, cap-poly(A) synergism, and initiation factors to nematode translation in an Ascaris suum embryo cell-free system. J Biol Chem. 2004. 279:45573–45585.
Article19. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007. 23:2947–2948.
Article20. Liddell S, Knox DP. Extracellular and cytoplasmic Cu/Zn superoxide dismutases from Haemonchus contortus. Parasitology. 1998. 116(Pt 4):383–394.
Article21. LoVerde PT, Carvalho-Queiroz C, Cook R. Vaccination with antioxidant enzymes confers protective immunity against challenge infection with Schistosoma mansoni. Mem Inst Oswaldo Cruz. 2004. 99:5 Suppl 1. 37–43.
Article22. McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000. 16:404–405.
Article23. Muleke CI, Ruofeng Y, Lixin X, Xinwen B, Xiangrui L. Cloning and sequence analysis of Haemonchus contortus HC58cDNA. DNA Seq. 2007. 18:176–183.24. Newlands GF, Skuce PJ, Knox DP, Smith WD. Cloning and expression of cystatin, a potent cysteine protease inhibitor from the gut of Haemonchus contortus. Parasitology. 2001. 122(Pt 3):371–378.25. Newton SE, Munn EA. The development of vaccines against gastrointestinal nematode parasites, particularly Haemonchus contortus. Parasitol Today. 1999. 15:116–122.
Article26. Selkirk ME, Smith VP, Thomas GR, Gounaris K. Resistance of filarial nematode parasites to oxidative stress. Int J Parasitol. 1998. 28:1315–1332.
Article27. Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993. 215:213–219.
Article28. Stadtman TC. Selenocysteine. Annu Rev Biochem. 1996. 65:83–100.
Article29. Stover NA, Steele RE. Trans-spliced leader addition to mRNAs in a cnidarian. Proc Natl Acad Sci USA. 2001. 98:5693–5698.
Article30. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007. 24:1596–1599.
Article31. Tang L, Gounaris K, Griffiths C, Selkirk ME. Heterologous expression and enzymatic properties of a selenium-independent glutathione peroxidase from the parasitic nematode Brugia pahangi. J Biol Chem. 1995. 270:18313–18318.
Article32. Tang L, Smith VP, Gounaris K, Selkirk ME. Brugia pahangi: the cuticular glutathione peroxidase (gp29) protects heterologous membranes from lipid peroxidation. Exp Parasitol. 1996. 82:329–332.
Article33. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994. 22:4673–4680.
Article34. Tripp C, Frank RS, Selkirk ME, Tang L, Grieve MM, Frank GR, Grieve RB. Dirofilaria immitis: molecular cloning and expression of a cDNA encoding a selenium-independent secreted glutathione peroxidase. Exp Parasitol. 1998. 88:43–50.
Article35. Williams C, Xu L, Blumenthal T. SL1 trans splicing and 3'-end formation in a novel class of Caenorhabditis elegans operon. Mol Cell Biol. 1999. 19:376–383.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fractionation of DNases Specific to Haemonchus contortus Intestine by Phenyl Sepharose Column
- Selenium Status and Glutathione Peroxidase Activity in Korean Infants
- Effect of Antioxidants on the Incidence of 7, 12-dimethylbenzanthracene-induced Mammary Tumor in Rats
- Effect of Sodium Selenite on Metallothionem Induction by the Treatment of Mercuric Chloride to Rats
- Characterization of HC58cDNA, a putative cysteine protease from the parasite Haemonchus contortus