J Korean Med Sci.
2007 Aug;22(4):693-697. 10.3346/jkms.2007.22.4.693.
The Efficacy of the COMFORT Scale in Assessing Optimal Sedation in Critically Ill Children Requiring Mechanical Ventilation
- Affiliations
-
- 1Department of Pediatrics, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Korea. drpsj@amc.seoul.kr
- 2Department of Pharmacology, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Korea.
- KMID: 1127089
- DOI: http://doi.org/10.3346/jkms.2007.22.4.693
Abstract
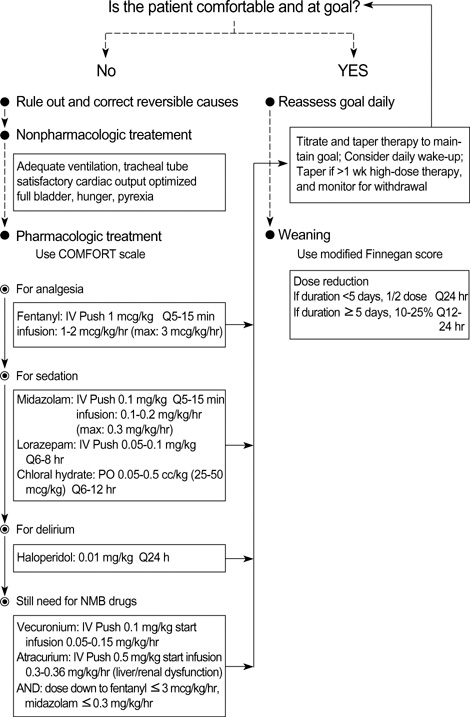
- Sedation is often necessary to optimize care for critically ill children requiring mechanical ventilation. If too light or too deep, however, sedation can cause significant adverse reactions, making it important to assess the degree of sedation and maintain its optimal level. We evaluated the efficacy of the COMFORT scale in assessing optimal sedation in critically ill children requiring mechanical ventilation. We compared 12 month data in 21 patients (intervention group), for whom we used the pediatric intensive care unit (PICU) sedation protocol of Asan Medical Center (Seoul, Korea) and the COMFORT scale to maintain optimal sedation, with the data in 20 patients (control group) assessed before using the sedation protocol and the COMPORT scale. Compared with the control group, the intervention group showed significant decreases in the total usage of sedatives and analgesics, the duration of mechanical ventilation (11.0 days vs. 12.5 days) and PICU stay (15.0 days vs. 19.5 days), and the development of withdrawal symptoms (1 case vs. 7 cases). The total duration of sedation (8.0 days vs. 11.5 days) also tended to decrease. These findings suggest that application of protocol-based sedation with the COMPORT scale may benefit children requiring mechanical ventilation.
Keyword
MeSH Terms
-
Anesthetics, Intravenous/administration & dosage/therapeutic use
Child, Preschool
Conscious Sedation/methods/standards
Critical Care/*methods/standards
*Critical Illness
Female
Fentanyl/administration & dosage/therapeutic use
Humans
Hypnotics and Sedatives/administration & dosage/*therapeutic use
Infant
Infusions, Intravenous
Intensive Care Units/statistics & numerical data
Length of Stay
Male
Midazolam/administration & dosage/therapeutic use
*Respiration, Artificial
Treatment Outcome
Figure
Reference
-
1. Polaner DM. Sedation-analgesia in the pediatric intensive care unit. Pediatr Clin North Am. 2001. 48:695–714.
Article2. Bavdekar SB, Mahajan MD, Chandu KV. Analgesia and sedation in paediatric intensive care unit. J Postgrad Med. 1999. 45:95–102.3. American College of Critical Care Medicine of the Society of Critical Care Medicine. American Society of Health-System Pharmacists. American College of Chest Physicians. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Am J Health Syst Pharm. 2002. 59:150–178.4. Keenan SP. Sedation in the critical care unit. Critical Care Rounds. 2000. 1:13–17.5. De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Using and understanding sedation scoring systems: a systemic review. Intensive Care Med. 2000. 26:275–285.6. Vender JS, Szokol JW, Murphy GS, Nitsun M. Sedation, analgesia, and neuromuscular blockade in sepsis: an evidence-based review. Crit Care Med. 2004. 32:S554–S561.
Article7. Hall RI, Sandham D, Cardinal P, Tweeddale M, Moher D, Wang X, Anis AH. Propofol vs midazolam for ICU sedation: a Canadian multicenter randomized trial. Chest. 2001. 119:1151–1159.8. McCollam JS, O'Neill MG, Norcross ED, Byrne TK, Reeves ST. Continuous infusion of lorazepam, midazolam, and propofol for sedation of the critically ill surgery trauma patient: a prospective randomized comparison. Crit Care Med. 1999. 27:2454–2458.9. Kelly DF, Goodale DB, Williams J, Herr DL, Chappell ET, Rosner MJ, Jacobson J, Levy ML, Croce MA, Maniker AH, Fulda GJ, Lovett JV, Mohan O, Narayan RK. Propofol in the treatment of moderate and severe head injury: a randomized, prospective double-blinded pilot trial. J Neurosurg. 1999. 90:1042–1052.
Article10. Detriche O, Berre J, Massaut J, Vincent JL. The Brussels sedation scale: use of a simple clinical sedation scale can avoid excessive sedation in patients undergoing mechanical ventilation in the intensive care unit. Br J Anaesth. 1999. 83:698–701.
Article11. Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000. 342:1471–1477.
Article12. Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999. 27:2609–2615.
Article13. Kollef MH, Shapiro SD, Silver P, St. John RE, Prentice D, Sauer S, Ahrens TS, Shannon W, Baker-Clinkscale D. A randomized, controlled trial of protocol directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997. 25:567–574.14. Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996. 335:1864–1869.
Article15. Wood G, MacLeod B, Moffatt S. Weaning from mechanical ventilation: physician-directed versus a respiratory-therapist-directed protocol. Respir Care. 1995. 40:219–224.16. Cohen IL, Bari N, Strosberg MA, Weinberg PF, Wacksman RM, Millstein BH, Fein IA. Reduction of duration and cost of mechanical ventilation in an intensive care unit by use of a ventilatory management team. Crit Care Med. 1991. 19:1278–1284.
Article17. Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Pediatr Psychol. 1992. 17:95–109.
Article18. van Dijk M, de Boer JB, Koot HM, Tibboel D, Passchier J, Duivenvoorden HJ. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3-year-old infants. Pain. 2000. 84:367–377.
Article19. Crain N, Slonim A, Pollack MM. Assessing sedation in the pediatric intensive care unit by using BIS and the COMFORT scale. Pediatr Crit Care Med. 2002. 3:11–14.
Article20. lsta E, van Dijk M, Tibboel D, de Hoog M. Assessment of sedation levels in pediatric intensive care patients can be improved by using the COMFORT "behavior" scale. Pediatr Crit Care Med. 2005. 6:58–63.21. Marx CM, Smith PG, Lowrie LH, Hamlett KW, Ambuel B, Yamashita TS, Blumer JL. Optimal sedation of mechanically ventilated pediatric critical care patients. Crit Care Med. 1994. 22:163–170.
Article22. Yaster M, Kost-Byerly S, Berde C, Billet C. The management of opioid and benzodiazepine dependence in infants, children, and adolescents. Pediatrics. 1996. 98:135–140.23. Devlin JW, Holbrook AM, Fuller HD. The effect of ICU sedation guidelines and pharmacist interventions on clinical outcomes and drug cost. Ann Pharmacother. 1997. 31:689–695.
Article24. MacLaren R, Plamondon JM, Ramsay KB, Rocker GM, Patrick WD, Hall RI. A prospective evaluation of empiric versus protocol-based sedation and analgesia. Pharmacotherapy. 2000. 20:662–672.
Article25. Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000. 28:2122–2132.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sedation in the Critically Ill Patients
- The Comparative Study of Central Venous Pressure Measurements during Mechanical Ventilation and after Disconnection of Ventilation
- Home mechanical ventilation in children with chronic respiratory failure: a narrative review
- Effect of Sedatives on In-hospital and Long-term Mortality of Critically Ill Patients Requiring Extended Mechanical Ventilation for ≥ 48 Hours
- Efficacy and Safety of Fentanyl in Combination with Midazolam in Children on Mechanical Ventilation