J Korean Med Sci.
2011 Nov;26(11):1454-1460. 10.3346/jkms.2011.26.11.1454.
Relationships of Basal Level of Serum 17-Hydroxyprogesterone with that of Serum Androstenedione and Their Stimulated Responses to a Low Dose of ACTH in Young Adult Patients with Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea. chshinpd@snu.ac.kr
- KMID: 1123445
- DOI: http://doi.org/10.3346/jkms.2011.26.11.1454
Abstract
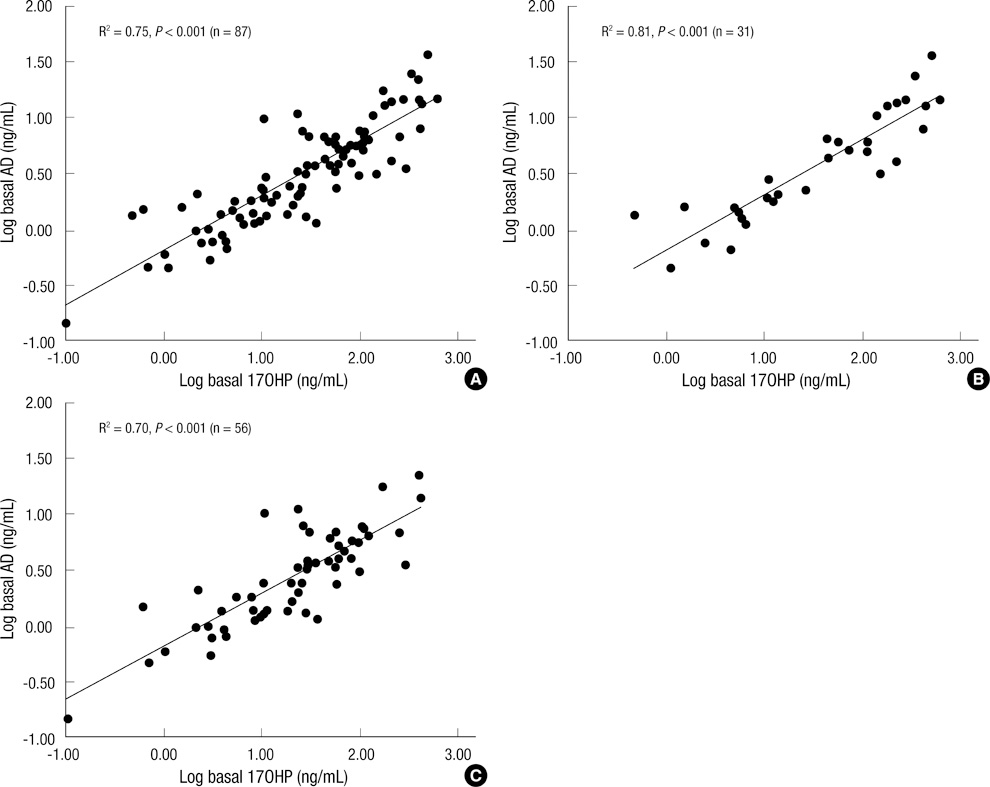
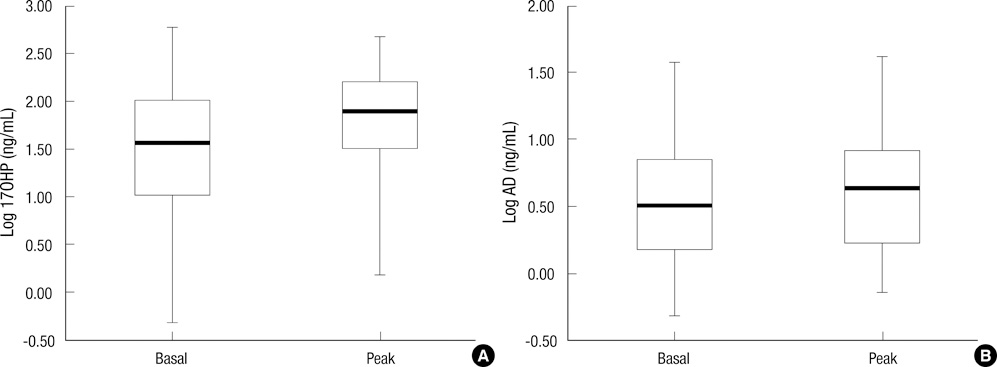
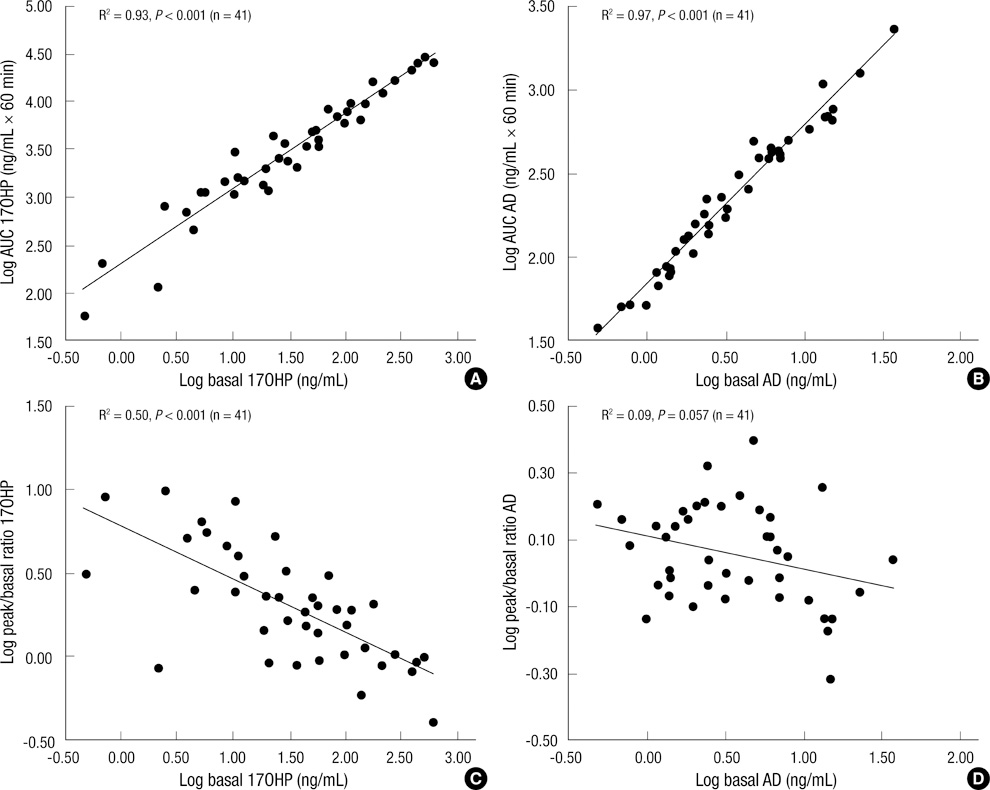
- A single measurement of serum 17alpha-hydroxyprogesterone (17OHP) level can be unreliable because of its marked diurnal variation. We investigated the relationship of serum level of 17OHP with that of androstenedione (AD), which shows a smaller diurnal variation. And we tested whether the responses of these two hormones to low-dose ACTH stimulation are correlated in patients with 21-hydroxylase deficiency. Baseline serum 17OHP and AD levels were measured in 87 patients and a low-dose ACTH stimulation test was performed in 41 patients. The basal 17OHP level correlated positively with the basal AD level independently of sex, type of 21-hydroxylase deficiency, and the time of day of blood sampling (n = 87, R2 = 0.75, P < 0.001). The area under the curve of 17OHP and AD correlated positively with their respective basal levels. The fold-change increase in 17OHP after ACTH injection correlated negatively with the basal 17OHP level, but that of AD did not correlate with the basal AD level. The random serum 17OHP level, used in the clinic, is a reliable guide and a low-dose ACTH stimulation test provides no extra benefit for assessing the treatment adequacy in patients with 21-hydroxylase deficiency.
MeSH Terms
Figure
Reference
-
1. Gardner R, Snaith AH. The urinary excretion of 17-hydroxysteroids in children. Arch Dis Child. 1958. 33:305–306.2. Cavallo A, Corn C, Bryan GT, Meyer WJ III. The use of plasma androstenedione in monitoring therapy of patients with congenital adrenal hyperplasia. J Pediatr. 1979. 95:33–37.3. Strott CA, Lipsett MB. Measurement of 17-hydroxyprogesterone in human plasma. J Clin Endocrinol Metab. 1968. 28:1426–1430.4. Barnes ND, Atherden SM. Diagnosis of congenital adrenal hyperplasia by measurement of plasma 17-hydroxyprogesterone. Arch Dis Child. 1972. 47:62–65.5. Horton R, Frasier SD. Androstenedione and its conversion to plasma testosterone in congenital adrenal hyperplasia. J Clin Invest. 1967. 46:1003–1009.6. Korth-Schutz S, Virdis R, Saenger P, Chow DM, Levine LS, New MI. Serum androgens as a continuing index of adequacy of treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1978. 46:452–458.7. Keenan BS, McNeel R, Barrett GN, Holcombe JH, Kirkland RT, Clayton GW. Plasma androgens in congenital adrenal hyperplasia: androstenedione concentration as an index of adrenal androgen suppression. J Lab Clin Med. 1979. 94:799–808.8. Strott CA, Yoshimi T, Lipsett MB. Plasma progesterone and 17-hydroxyprogesterone in normal men and children with congenital adrenal hyperplasia. J Clin Invest. 1969. 48:930–939.9. Colak R, Keleştimur F, Unlühizarci K, Bayram F, Sahin Y, Tutuş A. A comparison between the effects of low dose (1 microg) and standard dose (250 microg) ACTH stimulation tests on adrenal P450c17alpha enzyme activity in women with polycystic ovary syndrome. Eur J Endocrinol. 2002. 147:473–477.10. Unlühizarci K, Keleştimur F, Güven M, Bayram F, Colak R. The value of low dose (1 microg) ACTH stimulation test in the investigation of non-classic adrenal hyperplasia due to 11beta-hydroxylase deficiency. Exp Clin Endocrinol Diabetes. 2002. 110:381–385.11. Luboshitzky R, Ishai A, Shen-Or Z, Herer P. Evaluation of the pituitary-adrenal axis in hyperandrogenic women with polycystic ovary syndrome. Neuro Endocrinol Lett. 2003. 24:249–254.12. Conway DI, Anderson DC, Bu'lock DE. The steroid response to controlled adrenal stimulation in congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 1982. 16:215–226.13. Yi KH. Effect on final height of gonadotropin-releasing hormone agonist (GnRHa) in children with congenital adrenal hyperplasia. J Korean Soc Pediatr Endocrinol. 2005. 10:50–56.14. Charmandari E, Matthews DR, Johnston A, Brook CG, Hindmarsh PC. Serum cortisol and 17-hydroxyprogesterone interrelation in classic 21-hydroxylase deficiency: is current replacement therapy satisfactory? J Clin Endocrinol Metab. 2001. 86:4679–4685.15. Hindmarsh PC. Management of the child with congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009. 23:193–208.16. Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003. 349:776–788.17. Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC. Endocrine Society. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010. 95:4133–4160.18. Ilondo MM, Vanderschueren-Lodeweyckx M, Pizarro M, Vlietinck R, Malvaux P, Eggermont E, Eeckels R. Plasma levels of androgens and 17 alpha-OH-progesterone as an index of the adequacy of treatment in congenital adrenal hyperplasia. Horm Res. 1983. 18:175–185.19. Ghizzoni L, Bernasconi S, Virdis R, Vottero A, Ziveri M, Volta C, Iughetti L, Giovannelli G. Dynamics of 24-hour pulsatile cortisol, 17-hydroxyprogesterone, and androstenedione release in prepubertal patients with non-classic 21-hydroxylase deficiency and normal prepubertal children. Metabolism. 1994. 43:372–377.20. von Schnakenburg K, Bidlingmaier F, Knorr D. 17-hydroxyprogesterone, androstenedione, and testosterone in normal children and in prepubertal patients with congenital adrenal hyperplasia. Eur J Pediatr. 1980. 133:259–267.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Congenital Adrenal Hyperplasia due to 11beta-Hydroxylase Deficiency
- Congenital Adrenal Hyperplasia with 21-hydroxylase Deficiencies in Twins
- Hormone profile in patients with simple virilizing congenital adrenal hyperplasia due to 21-hydroxylase deficiency
- Genotype of Steroid 21-Hydroxylase Gene and Clinical Characteristics in Patients with Congenital Adrenal Hyperplasia 21-Hydroxylase Deficiency
- A Case of Congenital Adrenal Hyperlasia Misdiagnosed as Leydig Cell Tumor