Clin Orthop Surg.
2009 Jun;1(2):81-89. 10.4055/cios.2009.1.2.81.
The Increased Expression of Matrix Metalloproteinases Associated with Elastin Degradation and Fibrosis of the Ligamentum Flavum in Patients with Lumbar Spinal Stenosis
- Affiliations
-
- 1Department of Orthopaedic Surgery, The Catholic University of Korea School of Medicine, Uijeongbu, Korea. spinepjb@catholic.ac.kr
- 2Department of Pathology, The Catholic University of Korea School of Medicine, Uijeongbu, Korea.
- 3Department of Orthopaedic Surgery, Washington University School of Medicine, St. Louis, USA.
- KMID: 1122637
- DOI: http://doi.org/10.4055/cios.2009.1.2.81
Abstract
-
BACKGROUND: One of the characteristics of spinal stenosis is elastin degradation and fibrosis of the extracellular matrix of the ligamentum flavum. However, there have been no investigations to determine which biochemical factors cause these histologic changes. So we performed the current study to investigate the hypothesis that matrix metalloproteinases (MMPs), which possess the ability to cause extracellular matrix remodeling, may play a role as a mediator for this malady in the ligamentum flavum.
METHODS
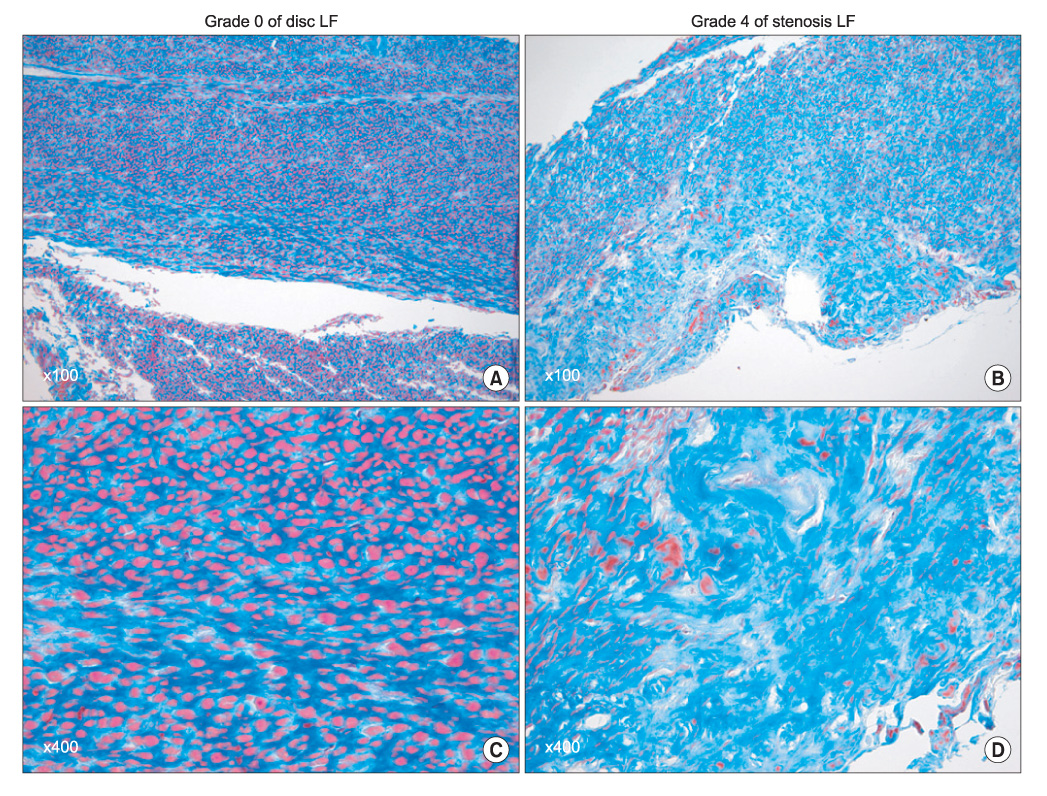
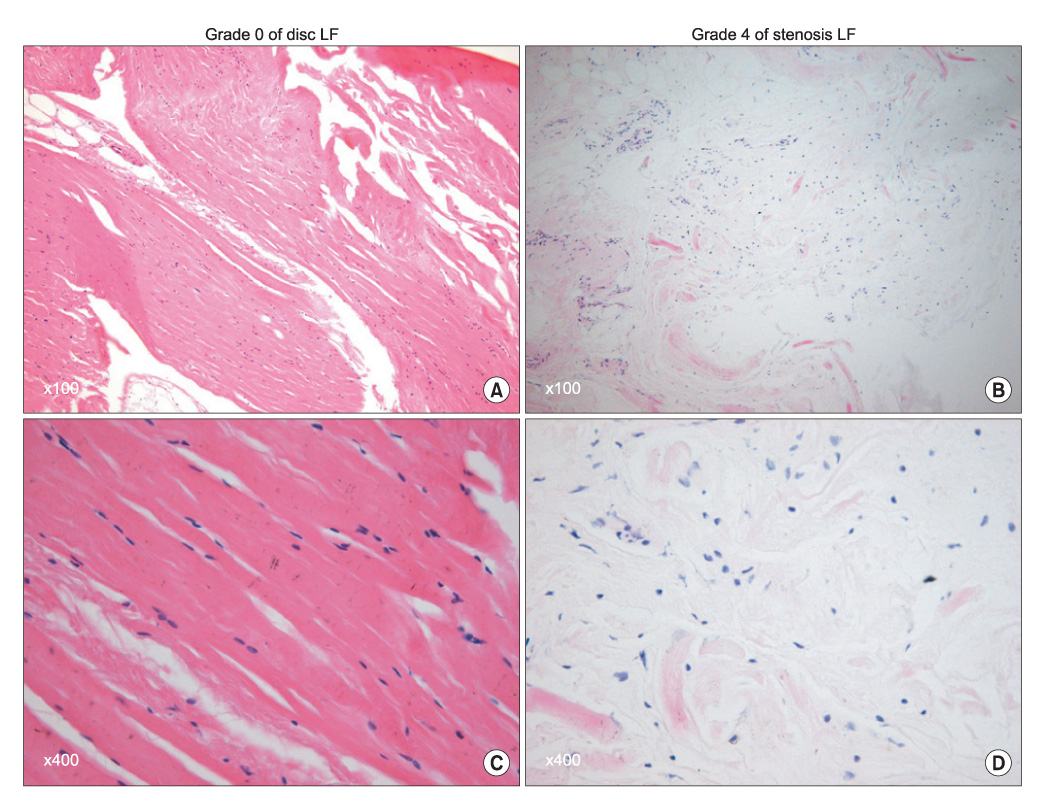
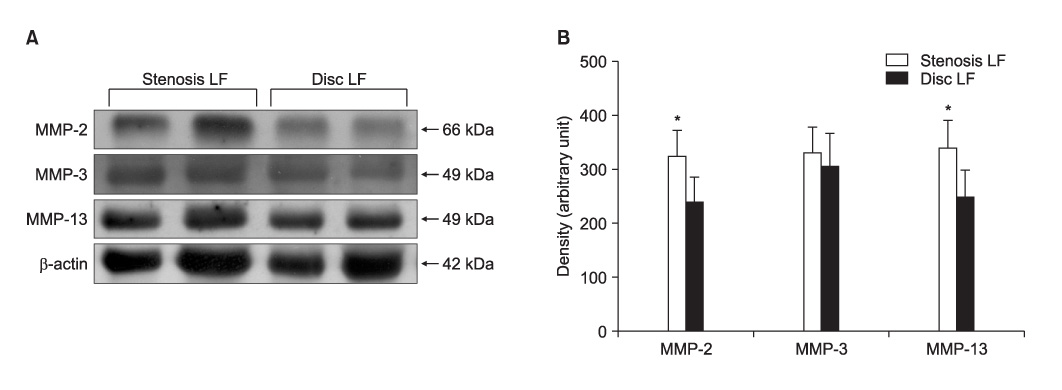
The ligamentum flavum specimens were surgically obtained from thirty patients with spinal stenosis, as well as from 30 control patients with a disc herniation. The extents of ligamentum flavum elastin degradation and fibrosis were graded (grade 0-4) with performing hematoxylin-eosin staining and Masson's trichrome staining, respectively. The localization of MMP-2 (gelatinase), MMP-3 (stromelysin) and MMP-13 (collagenase) within the ligamentum flavum tissue was determined by immunohistochemistry. The expressions of the active forms of MMP-2, MMP-3 and MMP-13 were determined by western blot analysis, and the blots were quantified using an imaging densitometer. The histologic and biochemical results were compared between the two conditions.
RESULTS
Elastin degradation and fibrosis of the ligamentum flavum were significantly more severe in the spinal stenosis samples than that in the disc herniation samples (3.14 +/- 0.50 vs. 0.55 +/- 0.60, p < 0.001; 3.10 +/- 0.57 vs. 0.76 +/- 0.52, p < 0.001, respectively). The expressions of the active form of MMPs were identified in all the ligamentum flavums of the spinal stenosis and disc herniation patients. The expressions of active MMP-2 and MMP-13 were significantly higher in the spinal stenosis samples than that in the disc herniation samples (both p < 0.05). The expression of active MMP-3 was slightly higher in the spinal stenosis samples than that in the disc herniation samples, but the difference was not statistically significant (p = 0.131). MMP-2, -3, and -13 were positively stained on the ligamentum flavum fibroblasts.
CONCLUSIONS
The current results suggest that the increased expression of active MMPs by the ligamentum flavum fibroblasts might be related to the elastin degradation and fibrosis of the ligamentum flavum in the patients who suffer with lumbar spinal stenosis.
Keyword
MeSH Terms
-
Aged
Blotting, Western
Elastin/*metabolism
Extracellular Matrix/metabolism/pathology
Female
Fibrosis
Humans
Immunohistochemistry
Ligamentum Flavum/*metabolism/pathology
*Lumbar Vertebrae
Male
Matrix Metalloproteinase 13/metabolism
Matrix Metalloproteinase 2/metabolism
Matrix Metalloproteinase 3/metabolism
Matrix Metalloproteinases/*metabolism
Middle Aged
Spinal Stenosis/*metabolism/pathology
Figure
Reference
-
1. Schrader PK, Grob D, Rahn BA, Cordey J, Dvorak J. Histology of the ligamentum flavum in patients with degenerative lumbar spinal stenosis. Eur Spine J. 1999. 8(4):323–328.
Article2. Yoshida M, Shima K, Taniguchi Y, Tamaki T, Tanaka T. Hypertrophied ligamentum flavum in lumbar spinal canal stenosis: pathogenesis and morphologic and immunohistochemical observation. Spine. 1992. 17(11):1353–1360.
Article3. Okuda T, Baba I, Fujimoto Y, et al. The pathology of ligamentum flavum in degenerative lumbar disease. Spine. 2004. 29(15):1689–1697.
Article4. Postacchini F, Gumina S, Cinotti G, Perugia D, DeMartino C. Ligamenta flava in lumbar disc herniation and spinal stenosis: light and electron microscopic morphology. Spine. 1994. 19(8):917–922.
Article5. Yahia H, Drouin G, Maurais G, Garzon S, Rivard CH. Degeneration of the human lumbar spine ligaments: an ultrastructural study. Pathol Res Pract. 1989. 184(4):369–375.6. Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999. 274(31):21491–21494.
Article7. Johnson LL, Dyer R, Hupe DJ. Matrix metalloproteinases. Curr Opin Chem Biol. 1998. 2(4):466–471.
Article8. Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991. 5(8):2145–2154.
Article9. Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF Jr. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989. 84(2):678–685.
Article10. Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004. 110(22):3480–3487.
Article11. Robert L, Robert AM, Jacotot B. Elastin-elastase-atherosclerosis revisited. Atherosclerosis. 1998. 140(2):281–295.
Article12. Patroi I, Annessi G, Girolomoni G. Mid-dermal elastolysis: a clinical, histologic, and immunohistochemical study of 11 patients. J Am Acad Dermatol. 2003. 48(6):846–851.
Article13. Chung AW, Au Yeung K, Sandor GG, Judge DP, Dietz HC, van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res. 2007. 101(5):512–522.
Article14. Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002. 11(4):308–320.
Article15. Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998. 23(14):1612–1626.
Article16. Sairyo K, Biyani A, Goel VK, et al. Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine. 2007. 32(11):E340–E347.
Article17. Park JB, Chang H, Lee JK. Quantitative analysis of transforming growth factor-beta 1 in ligamentum flavum of lumbar spinal stenosis and disc herniation. Spine. 2001. 26(21):E492–E495.
Article18. Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1. J Orthop Res. 2002. 20(6):1380–1386.
Article19. Fukuyama S, Nakamura T, Ikeda T, Takagi K. The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J Spinal Disord. 1995. 8(2):126–130.
Article20. Park JB, Lee JK, Park SJ, Riew KD. Hypertrophy of ligamentum flavum in lumbar spinal stenosis associated with increased proteinase inhibitor concentration. J Bone Joint Surg Am. 2005. 87(12):2750–2757.
Article21. Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001. 44(12):2777–2789.
Article22. Cawston T, Billington C, Cleaver C, et al. The regulation of MMPs and TIMPs in cartilage turnover. Ann N Y Acad Sci. 1999. 878:120–129.
Article23. Ishiguro N, Ito T, Ito H, et al. Relationship of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover: analyses of synovial fluid from patients with osteoarthritis. Arthritis Rheum. 1999. 42(1):129–136.
Article24. Booms P, Pregla R, Ney A, et al. RGD-containing fibrillin-1 fragments upregulate matrix metalloproteinase expression in cell culture: a potential factor in the pathogenesis of the Marfan syndrome. Hum Genet. 2005. 116(1-2):51–61.
Article25. Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000. 113(6):443–453.
Article26. Yoshihara Y, Nakamura H, Obata K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000. 59(6):455–461.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum Levels of TGF-beta1, TIMP-1 and TIMP-2 in Patients with Lumbar Spinal Stenosis and Disc Herniation
- Immunohistochemical Study of the Ligamentum Flavum in the Lumbar Spinal Stenosis
- Lumbar Spinal Stenosis Due to a Large Calcified Mass in the Ligamentum Flavum
- Focal Ligamentum Flavum Hypertrophy with Ochronotic Deposits: An Unusual Cause for Neurogenic Claudication in Alkaptonuria
- Effects of Expression of Matrix Metalloproteinases and Discoidin Domain Receptors in Ligamentum Flavum Fibrosis in Patients with Degenerative Lumbar Canal Stenosis