J Korean Med Sci.
2012 Feb;27(2):200-206. 10.3346/jkms.2012.27.2.200.
Oral Solubilized Ursodeoxycholic Acid Therapy in Amyotrophic Lateral Sclerosis: A Randomized Cross-Over Trial
- Affiliations
-
- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Neurology, Seoul Metropolitan Goverment Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Neurology, Clinical Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. kwoo@plaza.snu.ac.kr
- 4Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1120159
- DOI: http://doi.org/10.3346/jkms.2012.27.2.200
Abstract
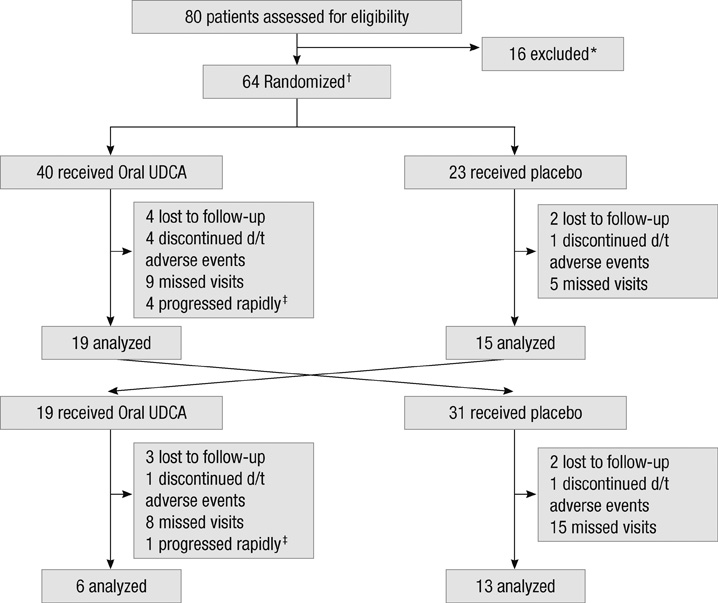
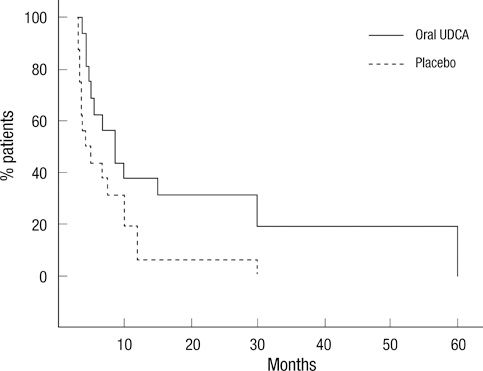
- To evaluate the efficacy and safety of ursodeoxycholic acid (UDCA) with oral solubilized formula in amyotrophic lateral sclerosis (ALS) patients, patients with probable or definite ALS were randomized to receive oral solubilized UDCA (3.5 g/140 mL/day) or placebo for 3 months after a run-in period of 1 month and switched to receive the other treatment for 3 months after a wash-out period of 1 month. The primary outcome was the rate of progression, assessed by the Appel ALS rating scale (AALSRS), and the secondary outcomes were the revised ALS functional rating scale (ALSFRS-R) and forced vital capacity (FVC). Fifty-three patients completed either the first or second period of study with only 16 of 63 enrolled patients given both treatments sequentially. The slope of AALSRS was 1.17 points/month lower while the patients were treated with UDCA than with placebo (95% CI for difference 0.08-2.26, P = 0.037), whereas the slopes of ALSFRS-R and FVC did not show significant differences between treatments. Gastrointestinal adverse events were more common with UDCA (P < 0.05). Oral solubilized UDCA seems to be tolerable in ALS patients, but we could not make firm conclusion regarding its efficacy, particularly due to the high attrition rate in this cross-over trial.
MeSH Terms
Figure
Cited by 1 articles
-
Update of Therapeutic Clinical Trials for Amyotrophic Lateral Sclerosis
Nam-Hee Kim, Min Oh Lee
Korean J Clin Neurophysiol. 2015;17(1):1-16. doi: 10.14253/kjcn.2015.17.1.1.
Reference
-
1. Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007. 369:2031–2041.2. Boillée S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006. 52:39–59.3. Hagey LR, Crombie DL, Espinosa E, Carey MC, Igimi H, Hofmann AF. Ursodeoxycholic acid in the Ursidae: biliary bile acids of bears, pandas, and related carnivores. J Lipid Res. 1993. 34:1911–1917.4. Leuschner U, Fischer H, Kurtz W, Güldütuna S, Hübner K, Hellstern A, Gatzen M, Leuschner M. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double-blind trial. Gastroenterology. 1989. 97:1268–1274.5. Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid 'mechanisms of action and clinical use in hepatobiliary disorders'. J Hepatol. 2001. 35:134–146.6. Rodrigues CM, Steer CJ. The therapeutic effects of ursodeoxycholic acid as an anti-apoptotic agent. Expert Opin Investig Drugs. 2001. 10:1243–1253.7. Rodrigues CM, Stieers CL, Keene CD, Ma X, Kren BT, Low WC, Steer CJ. Tauroursodeoxycholic acid partially prevents apoptosis induced by 3-nitropropionic acid: evidence for a mitochondrial pathway independent of the permeability transition. J Neurochem. 2000. 75:2368–2379.8. Solá S, Castro RE, Laires PA, Steer CJ, Rodrigues CM. Tauroursodeoxycholic acid prevents amyloid-beta peptide-induced neuronal death via a phosphatidylinositol 3-kinase-dependent signaling pathway. Mol Med. 2003. 9:226–234.9. Solá S, Castro RE, Kren BT, Steer CJ, Rodrigues CM. Modulation of nuclear steroid receptors by ursodeoxycholic acid inhibits TGF-beta1-induced E2F-1/p53-mediated apoptosis of rat hepatocytes. Biochemistry. 2004. 43:8429–8438.10. Ramalho RM, Borralho PM, Castro RE, Solá S, Steer CJ, Rodrigues CM. Tauroursodeoxycholic acid modulates p53-mediated apoptosis in Alzheimer's disease mutant neuroblastoma cells. J Neurochem. 2006. 98:1610–1618.11. Amaral JD, Castro RE, Solá S, Steer CJ, Rodrigues CM. p53 is a key molecular target of ursodeoxycholic acid in regulating apoptosis. J Biol Chem. 2007. 282:34250–34259.12. Duan WM, Rodrigues CM, Zhao LR, Steer CJ, Low WC. Tauroursodeoxycholic acid improves the survival and function of nigral transplants in a rat model of Parkinson's disease. Cell Transplant. 2002. 11:195–205.13. Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington's disease. Proc Natl Acad Sci U S A. 2002. 99:10671–10676.14. Rodrigues CM, Spellman SR, Solá S, Grande AW, Linehan-Stieers C, Low WC, Steer CJ. Neuroprotection by a bile acid in an acute stroke model in the rat. J Cereb Blood Flow Metab. 2002. 22:463–471.15. Rodrigues CM, Sola S, Nan Z, Castro RE, Ribeiro PS, Low WC, Steer CJ. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc Natl Acad Sci U S A. 2003. 100:6087–6092.16. Bellentani S. Immunomodulating and anti-apoptotic action of ursodeoxycholic acid: where are we and where should we go? Eur J Gastroenterol Hepatol. 2005. 17:137–140.17. Serviddio G, Pereda J, Pallardó FV, Carretero J, Borras C, Cutrin J, Vendemiale G, Poli G, Viña J, Sastre J. Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology. 2004. 39:711–720.18. Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994. 204:1–15.19. Park IH, Kim MK, Kim SU. Ursodeoxycholic acid prevents apoptosis of mouse sensory neurons induced by cisplatin by reducing P53 accumulation. Biochem Biophys Res Commun. 2008. 377:1025–1030.20. Thao TD, Ryu HC, Yoo SH, Rhee DK. Antibacterial and anti-atrophic effects of a highly soluble, acid stable UDCA formula in Helicobacter pylori-induced gastritis. Biochem Pharmacol. 2008. 75:2135–2146.21. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000. 1:293–299.22. Appel V, Stewart SS, Smith G, Appel SH. A rating scale for amyotrophic lateral sclerosis: description and preliminary experience. Ann Neurol. 1987. 22:328–333.23. Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995. 118:707–719.24. Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. BDNF ALS Study Group (Phase III). The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999. 169:13–21.25. Czaplinski A, Yen AA, Appel SH. Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS clinic population. J Neurol Neurosurg Psychiatry. 2006. 77:390–392.26. Lange DJ, Murphy PL, Diamond B, Appel V, Lai EC, Younger DS, Appel SH. Selegiline is ineffective in a collaborative double-blind, placebo-controlled trial for treatment of amyotrophic lateral sclerosis. Arch Neurol. 1998. 55:93–96.27. Grizzle JE. The two-period change-over design an its use in clinical trials. Biometrics. 1965. 21:467–480.28. Miller RG, Moore DH 2nd, Gelinas DF, Dronsky V, Mendoza M, Barohn RJ, Bryan W, Ravits J, Yuen E, Neville H, Ringel S, Bromberg M, Petajan J, Amato AA, Jackson C, Johnson W, Mandler R, Bosch P, Smith B, Graves M, Ross M, Sorenson EJ, Kelkar P, Parry G, Olney R. Western ALS Study Group. Phase III randomized trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology. 2001. 56:843–848.29. Traynor BJ, Zhang H, Shefner JM, Schoenfeld D, Cudkowicz ME. NEALS Consortium. Functional outcome measures as clinical trial endpoints in ALS. Neurology. 2004. 63:1933–1935.30. Parry GJ, Rodrigues CM, Aranha MM, Hilbert SJ, Davey C, Kelkar P, Low WC, Steer CJ. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic acid in patients with amyotrophic lateral sclerosis. Clin Neuropharmacol. 2010. 33:17–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amyotrophic Lateral Sclerosis Associated With CADASIL
- Syndrome of Progressive Bulbar Palsy in Amyotrophic Lateral Sclerosis: A Case Report
- Apraxia of Eyelid Closure and Motor Impersistence of Eyelid in a Patient with Amyotrophic Lateral Sclerosis
- Psychosocial Responses and Quality of Life among Amyotrophic Lateral Sclerosis Patients and Their Caregivers
- Effects of a combination of chenodeoxycholic acid and ursodeoxycholic acid for gallstone dissolution