Korean J Radiol.
2008 Dec;9(6):481-489. 10.3348/kjr.2008.9.6.481.
FDG PET/CT and Mediastinal Nodal Metastasis Detection in Stage T1 Non-Small Cell Lung Cancer: Prognostic Implications
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kyungs.lee@samsung.com
- 2Department of Thoracic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Division of Medical Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1118868
- DOI: http://doi.org/10.3348/kjr.2008.9.6.481
Abstract
OBJECTIVE
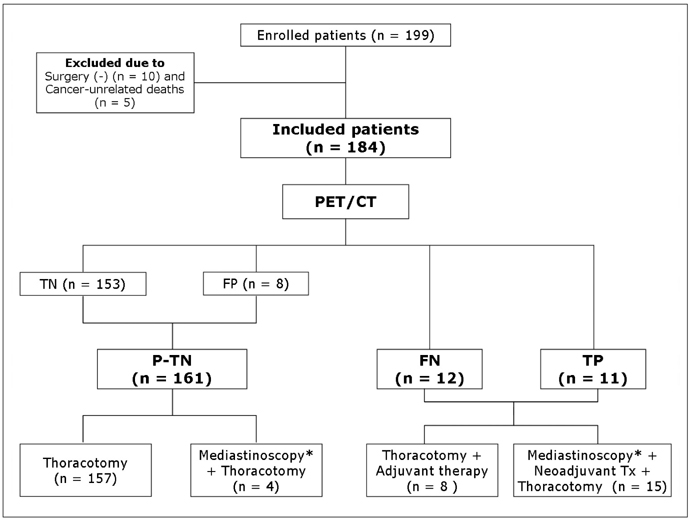
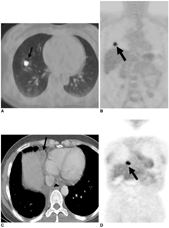
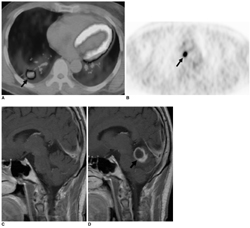
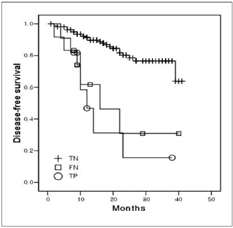
We aimed to compare the prognoses of patients with pathologically true negative (P-TN) N2 and PET/CT false negative (FN) results in stage T1 non-small cell lung cancer (NSCLC). MATERIALS AND METHODS: Our institutional review board approved this retrospective study with a waiver of informed consent. The study included 184 patients (124 men and 60 women; mean age, 59 years) with stage T1 NSCLC who underwent an integrated PET/CT and surgery. After estimating the efficacy of PET/CT for detecting N2 disease, we determined and compared disease-free survival (DFS) rates in three groups (P-TN [n = 161], PET/CT FN [n = 12], and PET/CT true positive [TP, n = 11]) using the Kaplan-Meier analysis and log-rank test. RESULTS: Pathologic N2 disease was observed in 23 (12%) patients. PET/CT had an N2 disease detection sensitivity of 48% (11 of 23 patients), a specificity of 95% (153 of 161), and an accuracy of 89% (164 of 184). The 3-year DFS rate in the PET/CT FN group (31%, 95% confidence interval [CI]; 13.6-48.0%) was similar to that of the TP group (16%, 95% CI; 1.7-29.5%) (p = 0.649), but both groups had significantly shorter DFS rates than the P-TN group (77%, 95% CI; 72.0-81.2%) (p < 0.001). CONCLUSION: The PET/CT shows a high specificity, but low sensitivity for detecting N2 disease in stage T1 NSCLC. Patients with PET/CT FN N2 disease have survival rates similar to PET/CT TP N2 disease patients, which are both substantially shorter than the survival rate of P-TN patients.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Carcinoma, Non-Small-Cell Lung/mortality/*radiography/*radionuclide imaging
Disease-Free Survival
Female
Fluorodeoxyglucose F18/diagnostic use
Humans
Lung Neoplasms/mortality/*radiography/*radionuclide imaging
Lymphatic Metastasis
Male
Mediastinum
Middle Aged
*Positron-Emission Tomography
Prognosis
Radiopharmaceuticals/diagnostic use
Sensitivity and Specificity
Survival Rate
*Tomography, X-Ray Computed
Figure
Reference
-
1. Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005. 236:1011–1019.2. Kim BT, Lee KS, Shim SS, Choi JY, Kwon OJ, Kim H, et al. Stage T1 non-small cell lung cancer: preoperative mediastinal nodal staging with integrated FDG PET/CT - a prospective study. Radiology. 2006. 241:501–509.3. Kim YK, Lee KS, Kim BT, Choi JY, Kim H, Kwon OJ, et al. Mediastinal nodal staging of nonsmall cell lung cancer using integrated 18F-FDG PET/CT in a tuberculosis-endemic country: diagnostic efficacy in 674 patients. Cancer. 2007. 109:1068–1077.4. Pearson FG, DeLarue NC, Ilves R, Todd TR, Cooper JD. Significance of positive superior mediastinal nodes identified at mediastinoscopy in patients with resectable cancer of the lung. J Thorac Cardiovasc Surg. 1982. 83:1–11.5. Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997. 111:1718–1723.6. Ahuja V, Coleman RE, Herndon J, Patz EF Jr. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer. 1998. 83:918–924.7. Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 2005. 23:1136–1143.8. Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004. 22:3255–3260.9. Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oguchi M, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002. 43:39–45.10. Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg. 2005. 130:151–159.11. Ohtsuka T, Nomori H, Watanabe K, Kaji M, Naruke T, Suemasu K, et al. Prognostic significance of [(18)F]fluorodeoxyglucose uptake on positron emission tomography in patients with pathologic stage I lung adenocarcinoma. Cancer. 2006. 107:2468–2473.12. van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007. 43:1392–1398.13. Vansteenkiste JF, Stroobants SG, Dupont PJ, De Leyn PR, Verbeken EK, Deneffe GJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol. 1999. 17:3201–3206.14. Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Kobayashi T, et al. Fluorine 18-tagged fluorodeoxyglucose positron emission tomographic scanning to predict lymph node metastasis, invasiveness, or both, in clinical T1 N0 M0 lung adenocarcinoma. J Thorac Cardiovasc Surg. 2004. 128:396–401.15. Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer. 2004. 45:19–27.16. Sagawa M, Higashi K, Sugita M, Ueda Y, Maeda S, Toga H, et al. Fluorodeoxyglucose uptake correlates with the growth pattern of small peripheral pulmonary adenocarcinoma. Surg Today. 2006. 36:230–234.17. Vesselle H, Freeman JD, Wiens L, Stern J, Nguyen HQ, Hawes SE, et al. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res. 2007. 13:3255–3263.18. Vesselle H, Turcotte E, Wiens L, Schmidt R, Takasugi JE, Lalani T, et al. Relationship between non-small cell lung cancer fluorodeoxyglucose uptake at positron emission tomography and surgical stage with relevance to patient prognosis. Clin Cancer Res. 2004. 10:4709–4716.19. Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000. 6:3837–3844.20. Flieder DB, Port JL, Korst RJ, Christos PJ, Levin MA, Becker DE, et al. Tumor size is a determinant of stage distribution in t1 non-small cell lung cancer. Chest. 2005. 128:2304–2308.21. Port JL, Kent MS, Korst RJ, Libby D, Pasmantier M, Altorki NK. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest. 2003. 124:1828–1833.22. Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg. 1994. 107:1087–1093.23. Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg. 2005. 129:87–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of PET in Staging Non-Small Cell Lung Cancer
- Accuracy of Nodal Staging with Integrated PET/CT Scanning in Non-small Cell Lung Cancer
- Significant Mismatch between FDG Uptake and Size after Chemotherapy in a Patient with Non-small Cell Lung Cancer
- Thoracic nodal staging in non-small cell lung cancer by FDG-PET
- The Ability of FDG Uptake Ratio and Glut-1 Expression to Predict Mediastinal Lymph Node Metastasis in Resected Non-small Cell Lung Cancer