Korean J Radiol.
2006 Sep;7(3):162-172. 10.3348/kjr.2006.7.3.162.
Structural Brain Abnormalities in Juvenile Myoclonic Epilepsy Patients: Volumetry and Voxel-Based Morphometry
- Affiliations
-
- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sbhong@smc.samsung.co.kr
- 2Department of Biomedical Engineering, Hanyang University, Seoul, Korea.
- 3Department of Neurology, College of Medicine, Ewha Womans University, Seoul, Korea.
- 4Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1114348
- DOI: http://doi.org/10.3348/kjr.2006.7.3.162
Abstract
OBJECTIVE
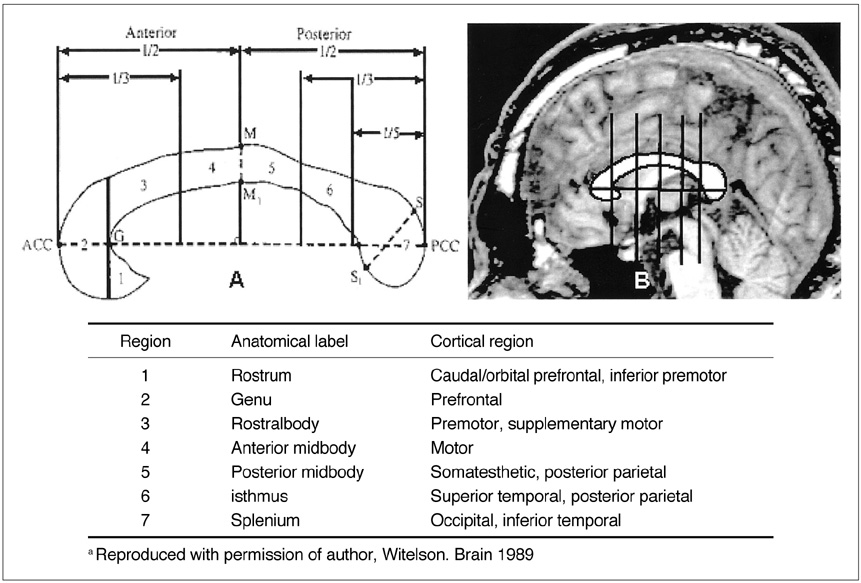
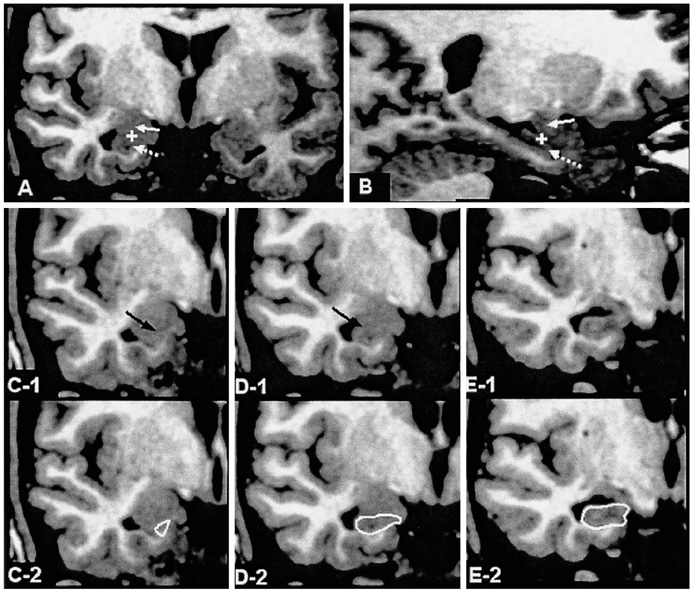
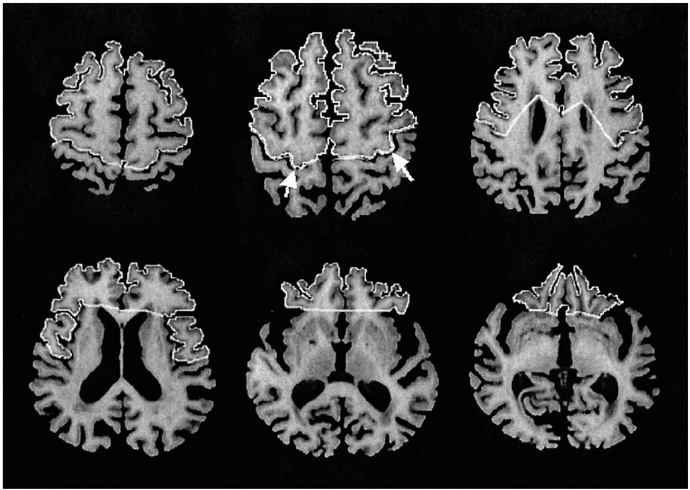
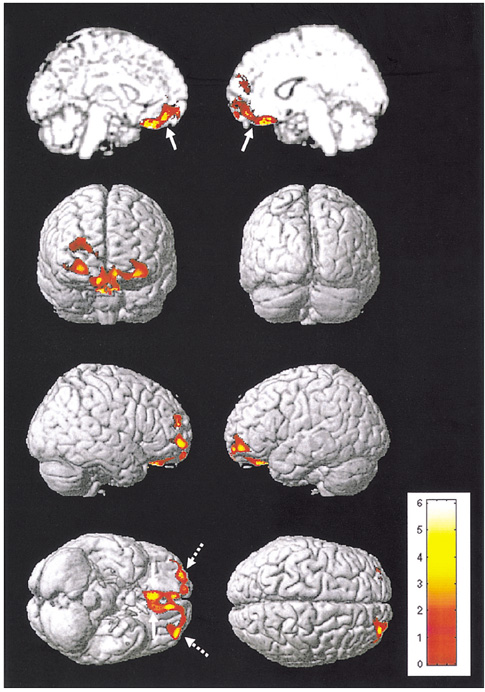
We aimed to find structural brain abnormalities in juvenile myoclonic epilepsy (JME) patients. MATERIALS AND METHODS: The volumes of the cerebrum, hippocampus and frontal lobe and the area of the corpus callosum's subdivisions were all semi-automatically measured, and then optimized voxel-based morphometry (VBM) was performed in 19 JME patients and 19 age/gender matched normal controls. RESULTS: The rostrum and rostral body of the corpus callosum and the left hippocampus were significantly smaller than those of the normal controls, whereas the volume of the JME's left frontal lobe was significantly larger than that of the controls. The area of the rostral body had a significant positive correlation with the age of seizure onset (r = 0.56, p = 0.012), and the volume of the right frontal lobe had a significant negative correlation with the duration of disease (r = -0.51, p = 0.025). On the VBM, the gray matter concentration of the prefrontal lobe (bilateral gyri rectus, anterior orbital gyri, left anterior middle frontal gyrus and right anterior superior frontal gyrus) was decreased in the JME group (corrected p < 0.05). CONCLUSION: The JME patients showed complex structural abnormalities in the corpus callosum, frontal lobe and hippocampus, and also a decreased gray matter concentration of the prefrontal region, which all suggests there is an abnormal neural network in the JME brain.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Relationship between Hippocampal Volume and Cognition in Patients with Chronic Primary Insomnia
Hyun Jin Noh, Eun Yeon Joo, Sung Tae Kim, So Mee Yoon, Dae Lim Koo, Daeyoung Kim, Geun-Ho Lee, Seung Bong Hong
J Clin Neurol. 2012;8(2):130-138. doi: 10.3988/jcn.2012.8.2.130.
Reference
-
1. Janz D, Durner M. Engel J, Pedley TA, editors. Juvenile myoclonic epilepsy. Epilepsy: A Comprehensive Textbook. 1998. Philadelphia: Lippincott-Raven;2389–2400.2. Panayiotopoulos CP, Obeid T. Juvenile myoclonic epilepsy: an autosomal recessive disease. Ann Neurol. 1989. 25:440–443.3. Liu AW, Delgado-Escueta AV, Serratosa JM, Alonso ME, Medina MT, Gee MN, et al. Juvenile myoclonic epilepsy locus in chromosome 6p21.2-p11: linkage to convulsions and electroencephalography trait. Am J Hum Genet. 1995. 57:368–381.4. Durner M, Sander T, Greenberg DA, Johnson K, Beck-Mannagetta G, Janz D. Localization of idiopathic generalized epilepsy on chromosome 6p in families of juvenile myoclonic epilepsy patients. Neurology. 1991. 41:1651–1655.5. Elmslie FV, Rees M, Williamson MP, Kerr M, Kjeldsen MJ, Pang KA, et al. Genetic mapping of a major susceptibility locus for juvenile myoclonic epilepsy on chromosome 15q. Hum Mol Genet. 1997. 6:1329–1334.6. Santiago-Rodriguez E, Harmony T, Fernandez-Bouzas A, Hernandez-Balderas A, Martinez-Lopez M, Graef A, et al. Source analysis of polyspike and wave complexes in juvenile myoclonic epilepsy. Seizure. 2002. 11:320–324.7. Savic I, Lekvall A, Greitz D, Helms G. MR spectroscopy shows reduced frontal lobe concentration of N-acetyl aspartate in patients with juvenile myoclonic epilepsy. Epilepsia. 2000. 41:290–296.8. Devinsky O, Gershengorn J, Brown E, Perrine K, Vazquez B, Luciano D. Frontal function in juvenile myoclonic epilepsy. Neuropsychiatry Neuropsychol Behav Neurol. 1997. 10:243–246.9. Koepp MJ, Richardson MP, Brooks DJ, Cunningham VJ, Duncan JS. Central benzodiazepine/gamma-aminobutyric acid A receptors in idiopathic generalized epilepsy: an [11C]-flumazenil positron emission tomography study. Epilepsia. 1997. 38:1089–1097.10. Swartz BE, Simpkins F, Halgren E, Mandelkern M, Brown C, Krisdakumtorn T, et al. Visual working memory in primary generalized epilepsy: an 18FDG-PET study. Neurology. 1996. 47:1203–1212.11. Gelisse P, Genton P, Samuelian JC, Thomas P, Bureau M. Psychiatric disorders in juvenile myoclonic epilepsy. Rev Neurol. 2001. 157:297–302.12. Gelisse P, Genton P, Raybaud C, Thomas P, Dravet C. Structural brain lesions do not influence the prognosis of juvenile myoclonic epilepsy. Acta Neurol Scand. 2000. 102:188–191.13. Woermann FG, Sisodiya SM, Free SL, Duncan JS. Quantitative MRI in patients with idiopathic generalized epilepsy. Evidence of widespread cerebral structural changes. Brain. 1998. 121:1661–1667.14. Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999. 122:2101–2108.15. Woermann FG, Free SL, Koepp MJ, Ashburner J, Duncan JS. Voxel-by-voxel comparison of automatically segmented cerebral gray matter-A rater-independent comparison of structural MRI in patients with epilepsy. Neuroimage. 1999. 10:373–384.16. Jack CR Jr, Bentley MD, Twomey CK, Zinsmeister AR. MR imaging-based volume measurements of the hippocampal formation and anterior temporal lobe: validation studies. Radiology. 1990. 176:205–209.17. Johannessen CU. Mechanisms of action of valproate: a commentatory. Neurochem Int. 2000. 37:103–110.18. Chapman AG, Riley K, Evans MC, Meldrum BS. Acute effects of sodium valproate and gamma-vinyl GABA on regional amino acid metabolism in the rat brain: incorporation of 2-[14C]glucose into amino acids. Neurochem Res. 1982. 7:1089–1105.19. DeLong MR. Kandal ER, Schwartz JH, Jessell TM, editors. The basal ganglia. Principles of neural science. 2000. 4th ed. New York: McGraw-Hill;853–867.20. Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989. 30:389–399.21. Witelson FS. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989. 112:799–835.22. Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. 1991. New York: Springer-Verlag.23. Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997. 120:141–157.24. Aylward EH, Anderson NB, Bylsma FW, Wagster MV, Barta PE, Sherr M, et al. Frontal lobe volume in patients with Huntington's disease. Neurology. 1998. 50:252–258.25. Arndt S, Swayze V, Cizadlo T, O'Leary D, Cohen G, Yuh WT, et al. Evaluating and validating two methods for estimating brain structure volumes: tessellation and simple pixel counting. Neuroimage. 1994. 1:191–198.26. Arndt S, Cohen G, Alliger RJ, Swayze VW 2nd, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res. 1991. 40:79–89.27. Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993. 34:71–75.28. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001. 14:21–36.29. Moses P, Courchesne E, Stiles J, Trauner D, Egaas B, Edwards E. Regional size reduction in the human corpus callosum following pre- and perinatal brain injury. Cereb Cortex. 2000. 10:1200–1210.30. Pelletier J, Suchet L, Witjas T, Habib M, Guttmann CR, Salamon G, et al. A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol. 2001. 58:105–111.31. Hampel H, Teipel SJ, Alexander GE, Horwitz B, Teichberg D, Schapiro MB, et al. Corpus callosum atrophy is a possible indicator of region- and cell type-specific neuronal degeneration in Alzheimer disease: a magnetic resonance imaging analysis. Arch Neurol. 1998. 55:193–198.32. Teipel SJ, Hampel H, Pietrini P, Alexander GE, Horwitz B, Daley E, et al. Region-specific corpus callosum atrophy correlates with the regional pattern of cortical glucose metabolism in Alzheimer disease. Arch Neurol. 1999. 56:467–473.33. Meschaks A, Lindstrom P, Halldin C, Farde L, Savic I. Regional reductions in serotonin 1A receptor binding in juvenile myoclonic epilepsy. Arch Neurol. 2005. 62:946–950.34. Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004. 161:99–108.35. Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005. 26:1271–1274.36. Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, et al. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002. 17:657–669.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution of Cerebral Gray and White Matters in Juvenile Myoclonic Epilepsy: Voxel Based Morphometry
- Two Cases of Juvenile Myoclonic Epilepsy of Janz
- Radiomic Models for Diagnosing Juvenile Myoclonic Epilepsy Should Note Its Genetic Heterogeneity
- Morphological Changes in Narcolepsy
- Wolff-Parkinson-White Syndrome in a Patient with Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like Episodes Syndrome Mimicking Juvenile Myoclonic Epilepsy