Yonsei Med J.
2011 Sep;52(5):773-778. 10.3349/ymj.2011.52.5.773.
The Bacterial Protein Azurin Enhances Sensitivity of Oral Squamous Carcinoma Cells to Anticancer Drugs
- Affiliations
-
- 1Department of Oral Anatomy and Cell Biology, School of Dentistry, Pusan National University, Medical Research Institute, Pusan National University Hospital, Yangsan, Korea. ki91000m@pusan.ac.kr
- 2Department of Pediatric Dentistry, College of Dentistry, Pusan National University, Medical Research Institute, Pusan National University Hospital, Yangsan, Korea. shinkim@pusan.ac.kr
- KMID: 1108070
- DOI: http://doi.org/10.3349/ymj.2011.52.5.773
Abstract
- PURPOSE
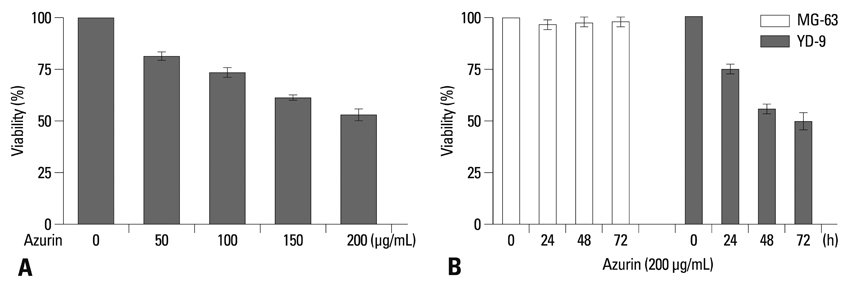
Surgical therapy is the primary treatment for oral cancer, but it can cause facial distortion. Therefore, if anticancer drugs are effective against oral cancer, they may be used preferentially. However, oral squamous carcinoma cells (OSCCs) are resistant to these drugs, so finding a way to enhance the sensitivity of these cells to anticancer drugs is important. The bacterial protein azurin is known to selectively enter cancer cells and induce apoptosis. In this study, we show the anticancer effect of azurin in OSCC.
MATERIALS AND METHODS
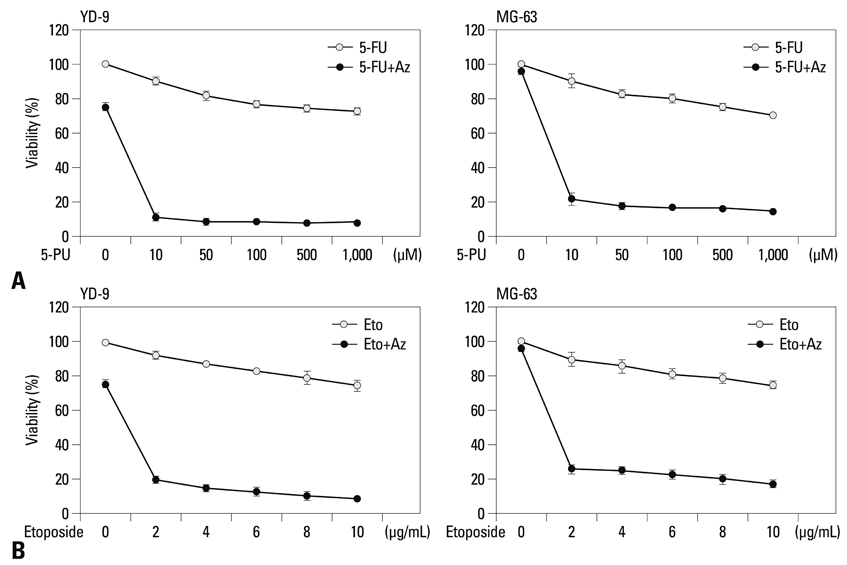
OSCC cell line (YD-9) was subjected to azurin treatment. Cell viability, morphology and protein expression levels were monitored after treatment of azurin. Cells were also subjected to combination treatment of azurin with either 5-fluorouracil or etopside.
RESULTS
Azurin-treated cells showed decreased cell viability accompanied by apoptotic phenotypes including morphological change, DNA breakage, and increases in p53 and cyclin B1 protein levels. Combination treatment of azurin with other anti-tumor agents caused an increase in sensitivity to anticancer drugs in azurin-treated YD-9 cells.
CONCLUSION
Azurin has a strong synergistic anticancer effect on oral cancer cells when it is used along with anticancer drugs.
Keyword
MeSH Terms
-
Antineoplastic Agents/*administration & dosage
Apoptosis/drug effects
Azurin/*administration & dosage/genetics
Carcinoma, Squamous Cell/*drug therapy/metabolism/pathology
Cell Line, Tumor
Cyclin B1/metabolism
Drug Synergism
Etoposide/administration & dosage
Fluorouracil/administration & dosage
Humans
Mouth Neoplasms/*drug therapy/metabolism/pathology
Tumor Suppressor Protein p53/metabolism
Figure
Reference
-
1. Hooper SJ, Wilson MJ, Crean SJ. Exploring the link between microorganisms and oral cancer: a systematic review of the literature. Head Neck. 2009. 31:1228–1239.
Article2. Chen XX, Lai MD, Zhang YL, Huang Q. Less cytotoxicity to combination therapy of 5-fluorouracil and cisplatin than 5-fluorouracil alone in human colon cancer cell lines. World J Gastroenterol. 2002. 8:841–846.
Article3. Yamada T, Fialho AM, Punj V, Bratescu L, Gupta TK, Chakrabarty AM. Internalization of bacterial redox protein azurin in mammalian cells: entry domain and specificity. Cell Microbiol. 2005. 7:1418–1431.
Article4. Yamada T, Goto M, Punj V, Zaborina O, Chen ML, Kimbara K, et al. Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc Natl Acad Sci U S A. 2002. 99:14098–14103.
Article5. Punj V, Bhattacharyya S, Saint-Dic D, Vasu C, Cunningham EA, Graves J, et al. Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene. 2004. 23:2367–2378.
Article6. Yamada T, Goto M, Punj V, Zaborina O, Kimbara K, Das Gupta TK, et al. The bacterial redox protein azurin induces apoptosis in J774 macrophages through complex formation and stabilization of the tumor suppressor protein p53. Infect Immun. 2002. 70:7054–7062.
Article7. Yang DS, Miao XD, Ye ZM, Feng J, Xu RZ, Huang X, et al. Bacterial redox protein azurin induce apoptosis in human osteosarcoma U2OS cells. Pharmacol Res. 2005. 52:413–421.
Article8. Borgne A, Versteege I, Mahé M, Studeny A, Léonce S, Naime I, et al. Analysis of cyclin B1 and CDK activity during apoptosis induced by camptothecin treatment. Oncogene. 2006. 25:7361–7372.
Article9. Porter LA, Singh G, Lee JM. Abundance of cyclin B1 regulates gamma-radiation-induced apoptosis. Blood. 2000. 95:2645–2650.10. Porter LA, Cukier IH, Lee JM. Nuclear localization of cyclin B1 regulates DNA damage-induced apoptosis. Blood. 2003. 101:1928–1933.
Article11. Yoshiba S, Ito D, Nagumo T, Shirota T, Hatori M, Shintani S. Hypoxia induces resistance to 5-fluorouracil in oral cancer cells via G(1) phase cell cycle arrest. Oral Oncol. 2009. 45:109–115.
Article12. Gomez LA, de Las Pozas A, Reiner T, Burnstein K, Perez-Stable C. Increased expression of cyclin B1 sensitizes prostate cancer cells to apoptosis induced by chemotherapy. Mol Cancer Ther. 2007. 6:1534–1543.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant Azurin from Pseudomonas aeruginosa Induces Apoptotic Cell Death in Oral Squamous Carcinoma Cells
- Anticancer effects of D-pinitol in human oral squamous carcinoma cells
- Diagnostic problem of squamous papilloma and oral mucosa malignancy
- Determination of pretreatment serum CEA and SCCA in patients with oral squamous cell carcinoma
- Methanol extracts of Asarum sieboldii Miq. induces apoptosis via the caspase pathway in human FaDu hypopharynx squamous carcinoma cells